肠道共生原生动物通过塑造肺部免疫来决定呼吸系统疾病的预后
IF 45.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
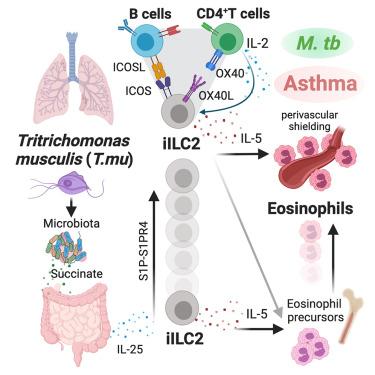
肠道微生物群影响宿主疾病结果的潜在机制尚不清楚。在这里,我们发现肠道共生原生动物,肌肉毛单胞菌(T.mu),远程塑造肺部免疫景观,以促进嗜酸性粒细胞对气道的血管周围屏蔽。肺特异性嗜酸性粒细胞增多需要肠源性炎性2组先天淋巴样细胞和肺常驻T细胞和B细胞之间的三方免疫网络。这个网络加剧了过敏性气道炎症的严重程度,同时阻碍了肺结核分枝杆菌的全身传播。严重变应性哮喘患者痰液中原生动物DNA序列的鉴定进一步强调了共生原生动物与人类疾病的相关性。总的来说,这些发现表明,一种共生原生动物通过肠道运作的肺免疫网络调节肺免疫,在对环境气道过敏原和肺部感染的反应中促进有益和有害的疾病结果。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A gut commensal protozoan determines respiratory disease outcomes by shaping pulmonary immunity
The underlying mechanisms used by the intestinal microbiota to shape disease outcomes of the host are poorly understood. Here, we show that the gut commensal protozoan, Tritrichomonas musculis (T.mu), remotely shapes the lung immune landscape to facilitate perivascular shielding of the airways by eosinophils. Lung-specific eosinophilia requires a tripartite immune network between gut-derived inflammatory group 2 innate lymphoid cells and lung-resident T cells and B cells. This network exacerbates the severity of allergic airway inflammation while hindering the systemic dissemination of pulmonary Mycobacterium tuberculosis. The identification of protozoan DNA sequences in the sputum of patients with severe allergic asthma further emphasizes the relevance of commensal protozoa in human disease. Collectively, these findings demonstrate that a commensal protozoan tunes pulmonary immunity via a gut-operated lung immune network, promoting both beneficial and detrimental disease outcomes in response to environmental airway allergens and pulmonary infections.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


