氢演化过程中金/电解质界面的稳定性:循环质谱伏安法研究
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
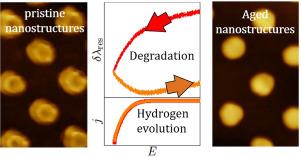
Stability of the Au/electrolyte interface during hydrogen evolution: A Cyclic Plasmo-Voltammetry study
Metal-electrolyte interfaces are dynamic entities, the potential and electrolyte dependent mobility of the metal atoms leading to surface restructuring with possible dissolution and degradation. In this work, we investigate the stability of the Au/aqueous electrolyte interface with in situ differential Cyclic Plasmo-Voltammetry (dCPV), augmented by ex situ atomic force microscopy and finite differential time domain simulations. We demonstrate that even the onset of hydrogen evolution is accompanied by pronounced morphological changes of the interface which are by far more prominent than those occurring during Au oxidation and reduction. Furthermore, the stability of the interface heavily depends on pH, the degradation of the electrode being considerably stronger in acidic than in neutral electrolyte. In addition, a clear hydrogen adsorption peak was observed in neutral electrolytes during the cathodic scan, which was more pronounced on a freshly prepared Au electrode than on an aged one. The measured dCPVs in acidic and neutral electrolytes can be explained consistently assuming that (1) adsorbed hydrogen is absorbed into the subsurface region of the Au electrode once HER starts; its subsequent removal as molecular hydrogen causes morphological changes; (2) in the presence of metal cations, adsorbed hydrogen is stabilized through the formation of ternary metal hydrides on the gold surface that stabilize the surface Au-H bonds and hinder further absorption of H into the subsurface region as well as the release of hydrogen into the electrolyte.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


