甲醇溶解放线离子和放线扩增卟啉络合物的发展趋势
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
自 1992 年分离出一系列三价镧系texaphyrin 复合物以来,三价锕系元素膨胀卟啉复合物一直备受合成界关注。在这项工作中,我们利用相对论密度泛函理论进行了计算研究,以确定三价锕系元素离子(Ac3+ 至 Lr3+)如何在甲醇溶剂中与希夫碱扩增的卟啉大环相互作用,作为稳定化的另一种途径。通过对结构参数、电子结构、微溶环境稳定性和相对结合能的全面分析,我们了解到了最稳定的结构。报告了甲醇溶解离子在整个锕系元素系列中的结合和结构趋势,并指出早期和中期锕系元素在该系列中的收缩。Texaphyrin 以平面方式结合了锕系元素离子,而对于 alaskphyrin 而言,弯曲结构更为有利;这种弯曲导致了比计算出的 Texaphyrin 复合物更强的相互作用能。我们还表明,具有氧化还原活性的扩展卟啉配体能够通过电荷转移稳定化来容纳多种锕系元素。根据相对结合能和能量分解分析发现,texaphyrin 和 alaskaphyrin 都表现出强烈的与 Th 结合的偏好。本文章由计算机程序翻译,如有差异,请以英文原文为准。
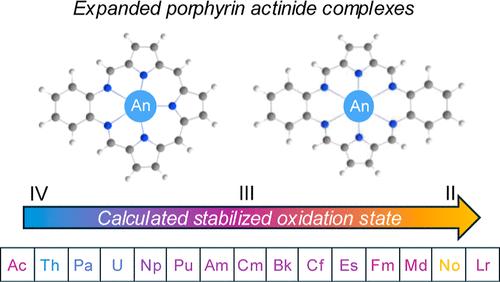
Trends in Methanol-Solvated Actinide Ions and Actinide Expanded Porphyrin Complexes
Trivalent actinide expanded porphyrin complexes have been of synthetic interest since the isolation of the series of trivalent lanthanide texaphyrin complexes in 1992, however, synthesis of these actinide-based complexes has not yet been achieved. In this work, a computational study with relativistic density functional theory was performed to determine how trivalent actinide ions (Ac3+ through Lr3+) interact with Schiff base expanded porphyrin macrocycles in a methanol solvent as an alternate pathway to stabilization. A thorough analysis of structural parameters, electronic structure, stability of microsolvation environments, and relative binding energies provided insight into the most stable structures. Trends in bonding and structure for the full actinide series are reported for methanol solvated ions, which reports actinide contraction along the series for early and mid actinides. Texaphyrin incorporates the actinide ions in a planar fashion, whereas for alaskphyrin a bent structure is more favorable; this bend results in stronger interaction energies than those calculated for the texaphyrin complexes. We also show that the redox-active expanded porphyrin ligands are able to accommodate a variety of actinides through charge transfer stabilization. Based on relative binding energy and energy decomposition analysis, it was found that texaphyrin and alaskaphyrin both exhibit a strong preference for binding to Th.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


