拼凑铜绿石多态性之谜:α- 和 β-Cu4O2(VO4)Cl 的合成、热膨胀和量子磁性α-和β-Cu4O2(VO4)Cl的合成、热膨胀和量子磁性
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
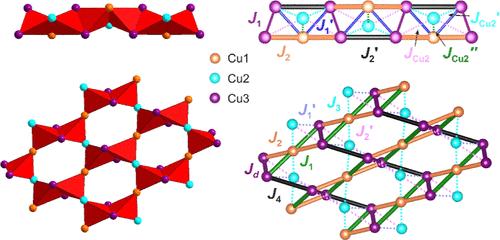
通过化学气相传输方法合成了两种新的二态自旋-1/2 量子磁体 α- 和 β-Cu4O2(VO4)Cl,该方法模拟了火山熔岩中矿物的形成。α-Cu4O2(VO4)Cl(1)是以[O2Cu4]4+ 1D 单棒为特征的共沸石矿物的纯钒酸盐类似物,而β-Cu4O2(VO4)Cl(2)则采用了[O2Cu4]4+ 2D 层状拓扑的新结构类型。报告采用高温单晶 X 射线衍射法研究了 1 和 2 的热膨胀。利用 ab initio 计算,我们推断出存在反铁磁性的 Cu1-Cu3 单元,其强耦合在 200-400 K 量级,形成链(1)和层(2)。Cu2 原子与这些单元的耦合很弱。磁感应强度测量结果表明,即使在 300 K 时,顺磁行为也会出现偏差,从而证实了这种情况。此外,1 在 TN = 24 K 以下显示出反铁磁有序性,具有微弱的未补偿磁矩。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Assembling the Puzzle of Coparsite Polymorphism: Synthesis, Thermal Expansion, and Quantum Magnetism of α- and β-Cu4O2(VO4)Cl
Two new dimorphic spin–1/2 quantum magnets, α- and β-Cu4O2(VO4)Cl, were synthesized via a chemical vapor transport method that emulates mineral formation in volcanic fumaroles. α-Cu4O2(VO4)Cl (1) is a pure vanadate analogue of the coparsite mineral characterized by [O2Cu4]4+ 1D single rods, whereas β-Cu4O2(VO4)Cl (2) adopts a new structure type with the [O2Cu4]4+ 2D layered topology. The thermal expansions of both 1 and 2 studied by high-temperature single-crystal X-ray diffraction are reported. Using ab initio calculations, we infer the presence of antiferromagnetic Cu1–Cu3 units with strong couplings on the order of 200–400 K forming chains (1) and layers (2). The Cu2 atoms are weakly coupled to such units. Magnetic susceptibility measurements corroborate this scenario by showing deviations from the paramagnetic behavior even at 300 K. Moreover, 1 reveals an antiferromagnetic ordering below TN = 24 K with a weak uncompensated magnetic moment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


