邻苯二甲酸酯的串联裂解氧化反应具有高CO2选择性和低能耗
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
气态邻苯二甲酸酯(PAEs)污染物具有极高的环境暴露风险,严重威胁人类健康。我们开发了具有空间隔离裂解-氧化双活性位点的 CuO@Hol-HZSM-5 催化剂,用于邻苯二甲酸二(2-乙基己基)/二辛基/二丁酯(DEHP、DOP、DBP)的氧热解处理。DOP 是 DEHP 的异构体,在 375 ℃ 和 20000 h-1 条件下,二者的转化率和二氧化碳选择性均达到约 99%。分子尺寸较小的 DBP 即使在低温 350 ℃ 和高速 30000 h-1 的条件下,也能实现与之相当的转化率和二氧化碳选择性。DFT 研究、原位 DRIFTS 和 GC-MS 揭示了布氏酸位点和氧化活性位点对 DEHP 分解的协同作用。反应过程由 CuO@Hol-HZSM-5 表面上的质子酸催化水解启动,生成邻苯二甲酸和 2-乙基己醇作为关键中间产物。2- 乙基己醇在质子酸催化下发生串联脱水-β-裂解反应,生成低分子量的烯烃,如乙烯和丙烯。邻苯二甲酸直接脱羧反应或邻苯二甲酸酐水合脱羧反应产生的低分子量烯烃和苯随后在 CuO 纳米粒子上转化为 CO2 和 H2O。开发了用于 DEHP 解吸气体氧热解处理的中试规模设备。裂解-氧化反应产生的放热能量通过一个集成热交换器被重新利用,以预热用于热解吸附的新鲜空气。出口冷凝液的化学需氧量为 26.86-45.96 mg/L,低于国家废水排放标准。热脱附-氧热解工艺的运行成本为 16.77 欧元/吨吸附剂,优于传统方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
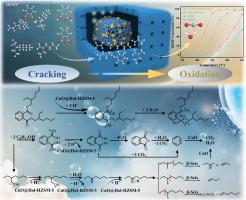

The tandem cracking-oxidation of phthalate esters with high CO2 selectivity and reduced energy cost
Gaseous phthalate ester (PAEs) pollutants pose a significantly high risk of environmental exposure and present a serious threat to human health. We developed the CuO@Hol-HZSM-5 catalyst with spatially isolated cracking-oxidation dual active sites for the oxypyrolysis treatment of di(2-ethylhexyl)/dioctyl/dibutyl phthalates (DEHP, DOP, DBP). DOP is an isomer of DEHP, and both of them exhibit the conversion and CO2 selectivity of ca. 99 % at 375 ℃ and 20000 h−1. DBP with smaller molecular size achieves comparable conversion and CO2 selectivity even at a low temperature 350 °C and high space velocity 30000 h−1. DFT studies, in-situ DRIFTS and GC–MS reveal the synergy of Brønsted acid sites and oxidation active sites for the DEHP decomposition. The reaction process is initiated by protonic acid-catalyzed hydrolysis over the CuO@Hol-HZSM-5 surface, yielding phthalic acid and 2-ethylhexanol as key intermediates. 2-Ethylhexanol undergoes a protonic acid-catalyzed tandem dehydration-β-scission reaction, generating low-molecular-weight alkenes such as ethylene and propylene. Low-molecular-weight alkenes and benzene from direct decarboxylation of phthalic acid or hydration-decarboxylation of phthalic anhydride are then converted to CO2 and H2O over CuO nanoparticles. Pilot-scale equipment was developed for the oxypyrolysis treatment of DEHP desorption gas. The exothermic energy from the cracking-oxidation reaction is re-utilized via an integrated heat exchanger to preheat the fresh air for thermal desorption. The COD of the outlet condensates was 26.86–45.96 mg/L, which is below the national wastewater discharge standards. The operation cost of the thermal desorption-oxypyrolysis process is €16.77/tonsorbent, which outperforms the traditional method.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


