CTLA-4检查点疗法的疗效依赖于IL-21信号介导PD-1+CD8+ T细胞的细胞毒性重编程
IF 27.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
抗程序性细胞死亡蛋白1 (PD-1)和抗细胞毒性T淋巴细胞相关蛋白4 (CTLA-4)治疗的作用机制尚不完全清楚。在这里,通过免疫分析对晚期黑色素瘤患者PD-1+CD8+ T (TResp)细胞群的反应,我们确定了TResp细胞对联合治疗的差异编程,从耗尽到更强的细胞毒性效应程序。抗pd -1单药治疗不会出现这种效果。单细胞转录组和T细胞受体库分析用于鉴定扩增的PD-1+CD8+ T细胞克隆的效应物编程改变,这些细胞克隆具有不同的调节使用,STAT1和STAT3利用以及与白细胞介素(IL)-21信号通路相关的抗肿瘤特异性联合和抗ctla -4单药治疗。在Il21r−缺乏或抗il -21受体阻断的B16F10黑色素瘤模型中,CTLA-4阻断的治疗效果丧失。总之,这些结果显示了IL-21信号传导到TResp对基于ctla -4的检查点治疗至关重要,并突出了与抗pd -1单药治疗的主要信号传导差异。本文章由计算机程序翻译,如有差异,请以英文原文为准。
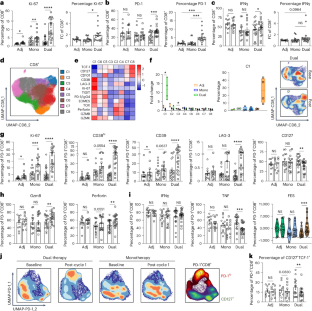

Efficacy of CTLA-4 checkpoint therapy is dependent on IL-21 signaling to mediate cytotoxic reprogramming of PD-1+CD8+ T cells
The mechanisms underlying the efficacy of anti-programmed cell death protein 1 (PD-1) and anti-cytotoxic T lymphocyte-associated protein 4 (CTLA-4) therapy are incompletely understood. Here, by immune profiling responding PD-1+CD8+ T (TResp) cell populations from patients with advanced melanoma, we identified differential programming of TResp cells in response to combination therapy, from an exhausted toward a more cytotoxic effector program. This effect does not occur with anti-PD-1 monotherapy. Single-cell transcriptome and T cell receptor repertoire analysis was used to identify altered effector programming of expanding PD-1+CD8+ T cell clones with distinct regulon usage, STAT1 and STAT3 utilization and antitumor specificity connected to interleukin (IL)-21 signaling in combination and anti-CTLA-4 monotherapy. Therapeutic efficacy of CTLA-4 blockade was lost in B16F10 melanoma models with either Il21r− deficiency or anti-IL-21 receptor blockade. Together, these results show how IL-21 signaling to TResp is critical for anti-CTLA-4-based checkpoint therapies and highlight major signaling differences to anti-PD-1 monotherapy. Here the authors dissect the contribution of IL-21 signaling to immune checkpoint-responding CD8+ T cells from mouse models and patients being treated for advanced melanoma.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


