N-Formyl Mirabegron 的鉴定、合成和表征:一种由药用辅料中的甲酸杂质形成的新降解产物。
IF 3
Q3 PHARMACOLOGY & PHARMACY
Advances in Pharmacological and Pharmaceutical Sciences
Pub Date : 2024-12-10
eCollection Date: 2024-01-01
DOI:10.1155/adpp/4971456
引用次数: 0
摘要
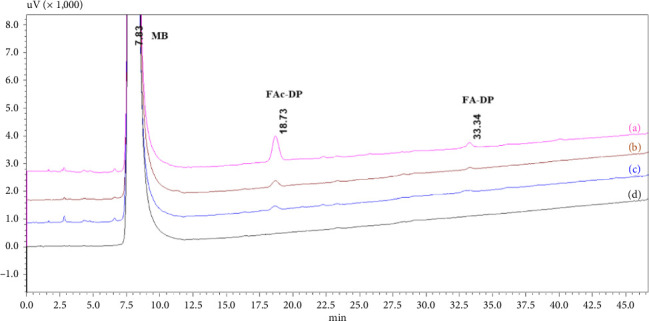
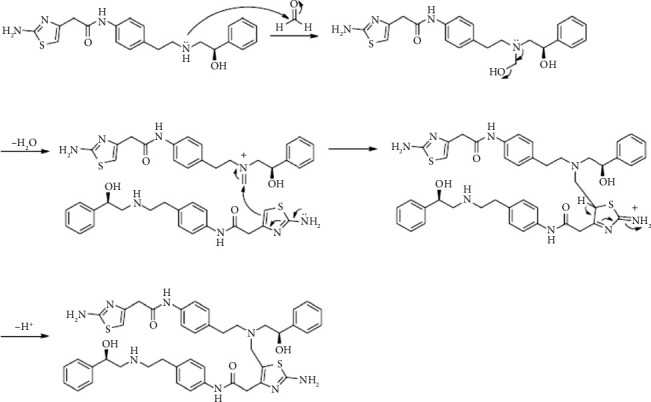
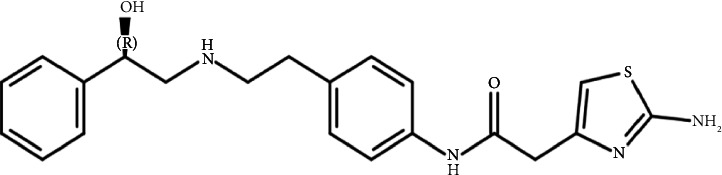
本研究证实了药用辅料中常见的活性杂质甲酸(FAc)对米拉米格隆(MB)稳定性的影响。mb -赋形剂相容性试验的研究表明,在55°C等温胁迫7天后,在0.2%的浓度下形成了一种新的降解产物facc - dp。采用液相色谱-质谱联用(LC-MS)、一维和二维核磁共振波谱(NMR)对FAc-DP进行了表征,并鉴定为n -甲酰基MB。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Identification, Synthesis, and Characterization of N-Formyl Mirabegron: A New Degradation Product Formed by Formic Acid Impurity in Pharmaceutical Excipients.
This study demonstrated the impact of formic acid (FAc), a common reactive impurity in pharmaceutical excipients, on the stability of mirabegron (MB). The investigation of MB-excipient compatibility tests revealed the formation of a new degradation product, FAc-DP, at 0.2% after 7 days of isothermal stress at 55°C. FAc-DP was synthesized and characterized as N-formyl MB using liquid chromatography-mass spectrometry (LC-MS) and both 1D and 2D nuclear magnetic resonance spectroscopy (NMR).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Advances in Pharmacological and Pharmaceutical Sciences
PHARMACOLOGY & PHARMACY-
CiteScore
4.30
自引率
3.60%
发文量
0
审稿时长
17 weeks
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


