揭示 CO 氧化反应中氧气对 Pt/CeO2 烧结机理的影响
IF 4.8
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
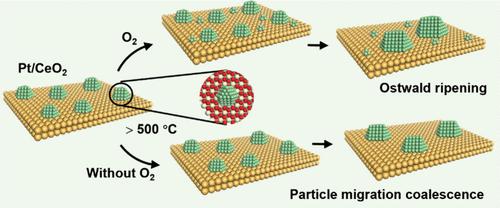
Unravelling the Effect of Oxygen on the Sintering Mechanism of Pt/CeO2 in the CO Oxidation Reaction
Sintering significantly contributes to the deactivation of supported metal catalysts under reaction conditions, influenced by various factors, including temperature, atmosphere, and metal–support interactions. The sintering mechanism under the reaction conditions remains complex and ambiguous. This study delves into the sintering behavior of platinum on CeO2 under CO oxidation conditions, mainly employing transmission electron microscopy to elucidate the effects of different gas components on the sintering mechanism at elevated temperatures. An atmosphere rich in oxygen promotes the sintering of Pt via the Ostwald ripening mechanism, characterized by the growth of larger nanoparticles at the expense of smaller ones. Conversely, sintering proceeds through particle migration and coalescence in the absence of oxygen, leading to the aggregation of nanoparticles. The obtained Pt/CeO2 catalysts exhibit enhanced catalytic performance after treatment with argon and stoichiometric reaction gases, contrasting with the deactivation observed under oxygen-rich conditions, which is attributed to varied Pt valence states. These findings provide insights into understanding the sintering mechanisms and inform the design of heterogeneous catalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


