通过镍光氧催化实现碳水化合物衍生物的位点选择性 O-芳基化
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
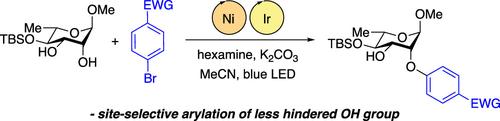
Site-Selective O-Arylation of Carbohydrate Derivatives through Nickel–Photoredox Catalysis
Site-selective O-arylations of glycoside-derived diols have been achieved by couplings with bromoarenes upon irradiation with blue LEDs in the presence of an iridium photocatalyst and a nickel complex. The use of hexamethylenetetramine (hexamine) in place of quinuclidine, along with the application of a methoxy-substituted 2,2′-bipyridine ligand, provided improvements in yield for this relatively challenging substrate class. Selective arylation took place at the less sterically hindered OH group, as determined by the substitution pattern and configuration of the glycoside substrate. Percent buried volume calculations were used to quantify the relative levels of steric hindrance at the two reactive sites.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


