镍光氧化还原/双催化氨基烷基镍转移半加氢反应的研究
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在催化转移加氢(TH)中使用胺是具有挑战性的,尽管它们作为氢源的潜在可用性。在这里,我们描述了一个光氧化还原/镍催化炔的TH通过中间氨基烷基镍。该反应成功地提供了功能化的(Z)-烯烃,如(homo)烯丙醚、醇和酰胺(Z/E =高达>;99:1),因此使用胺和Lindlar催化剂,该反应绕过了TH中底物范围的限制。机理研究表明,氨基烷基镍可能参与两种催化剂再生途径:(1)β-氢化物消除后的还原消除和(2)烯基镍的原脱金属。本文章由计算机程序翻译,如有差异,请以英文原文为准。
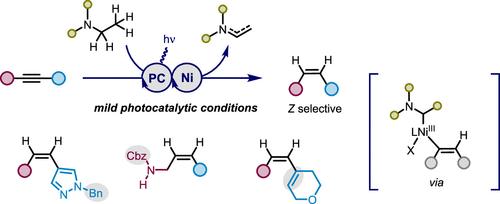
Nickel Photoredox/Dual-Catalyzed Transfer Semi-Hydrogenation of Alkynes via Aminoalkyl Nickel Species
Using amines in catalytic transfer hydrogenation (TH) is challenging, despite their potential availability as a hydrogen source. Here, we describe a photoredox/nickel-catalyzed TH of alkyne through an intermediary aminoalkyl Ni species. This reaction successfully provided functionalized (Z)-alkenes, such as (homo)allyl ethers, alcohols, and amides (Z/E = up to >99:1), and the reaction thus bypasses a limitation of substrate scope in TH using amine and a Lindlar catalyst. Mechanistic studies revealed that the aminoalkyl Ni species plausibly participates in two catalyst regeneration paths: (1) β-hydride elimination followed by reductive elimination and (2) protodemetalation from alkenyl NiI.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


