基因激活框架核酸靶向上调Sirtuin-1调节糖尿病骨质疏松治疗的骨免疫微环境
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
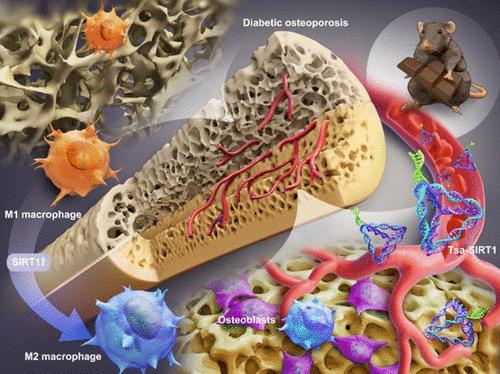
糖尿病性骨质疏松症是糖尿病的一种常见的慢性并发症,其特点是骨量减少,骨脆性增加,易骨折。这种情况的一个重要原因是由于长时间的高血糖导致成骨细胞稳态的破坏,这阻碍了骨再生和重塑。尽管糖尿病骨质疏松症很普遍,但目前尚无针对糖尿病骨质疏松症的有效治疗方法。近年来,小激活RNA (small-activating RNA, saRNA)疗法因其靶向性强、疗效高、副作用小等特点而备受关注。然而,RNA的固有特性,如结构不稳定、易降解和渗透性差,限制了它的应用。为了解决这些限制,开发了具有sirtuin-1 (SIRT1)基因激活功能的基因激活四面体框架核酸(tFNA),称为Tsa。Tsa具有rna保护作用,可有效穿透细胞膜上调SIRT1基因表达。在组织学水平上,Tsa治疗通过增加骨小梁密度和促进新骨形成来缓解糖尿病性骨质疏松症。在细胞水平上,它将巨噬细胞极化转向抗炎M2表型,同时抑制炎症M1表型,为成骨细胞创造有利的骨免疫微环境。在遗传水平上,Tsa激活SIRT1表达,使Acetyl-p65去乙酰化,阻断NF-κB通路,恢复骨免疫环境。总的来说,本研究展示了一种纳米药物“Tsa”,能够激活SIRT1并调节骨免疫环境,从而显示其在糖尿病骨质疏松症治疗中的巨大潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Gene-Activating Framework Nucleic Acid-Targeted Upregulating Sirtuin-1 to Modulate Osteoimmune Microenvironment for Diabetic Osteoporosis Therapeutics
Diabetic osteoporosis, a prevalent chronic complication of diabetes, is marked by reduced bone mass, increased bone fragility, and susceptibility to fractures. A significant cause of this condition is the disruption of osteoblastic homeostasis due to prolonged hyperglycemia, which impedes bone regeneration and remodeling. Despite its prevalence, no effective treatments specifically target diabetic osteoporosis. Recently, small-activating RNA (saRNA) therapy has attracted attention for its targeting capacity, high efficacy, and minimal side effects. However, RNA’s inherent properties, such as structural instability, susceptibility to degradation, and poor penetration, limit its applications. To address these limitations, a gene-activating tetrahedral framework nucleic acid (tFNA) with sirtuin-1 (SIRT1) gene activation function is developed, termed Tsa. Tsa exhibits an RNA-protecting effect and can effectively penetrate cell membranes to upregulate SIRT1 gene expression. At the histological level, Tsa treatment alleviates diabetic osteoporosis by increasing bone trabecular density and promoting new bone formation. At the cellular level, it switches macrophage polarization toward the anti-inflammatory M2 phenotype while inhibiting the inflammatory M1 phenotype, creating a favorable bone immune microenvironment for osteoblasts. At the genetic level, Tsa activates SIRT1 expression, which deacetylates Acetyl-p65 to block the NF-κB pathway and restore the osteoimmune environment. Overall, this research demonstrates a nanodrug “Tsa”, capable of activating SIRT1 and modulating the bone immune environment, thereby showcasing its immense potential for diabetic osteoporosis treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


