缺氧条件下,FTO通过m6A/YTHDF3/PDK1轴激活PD-L1促进乳腺癌的免疫抑制
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
导言改变的表观遗传学重编程使乳腺癌细胞能够适应缺氧压力。研究旨在确定脂肪量和肥胖相关蛋白(FTO)如何帮助乳腺癌细胞应对缺氧微环境,以及乳腺癌细胞抵抗肿瘤免疫的机制。方法利用临床样本分析FTO对乳腺癌进展的影响以及程序性细胞死亡蛋白1/程序性细胞死亡配体1(PD-1/PD-L1)免疫检查点抑制剂治疗的效果。利用MeRIP-seq和mRNA-seq分析缺氧条件下FTO调控的下游基因。利用 RIP 明确了 FTO 对 PDK1 的甲基化修饰调控。结果N6-甲基腺苷(m6A)去甲基化酶FTO被缺氧诱导因子1α(HIF-1α)转录激活。通过高通量测序,发现PDK1是FTO在缺氧条件下的潜在靶点。从机理上讲,过量表达FTO会减少PDK1 mRNA上的m6A修饰位点,阻止YTH结构域家族3(YTHDF3)识别并与这些位点结合,从而抑制PDK1 mRNA的降解。过量表达 PDK1 会激活 AKT/STAT3 通路,导致 PD-L1 表达增强。用 FB23 和 BX-912 靶向 FTO 和 PDK1-AKT 通路可抑制乳腺癌的生长,增强细胞毒性 T 淋巴细胞(CTL)的活性,并提高 PD-1/PD-L1 检查点抑制剂 Atezolizumab 的疗效。在缺氧条件下抑制 FTO 和 PDK1 可作为一种有前途的乳腺癌免疫治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
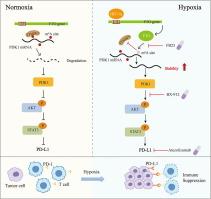
FTO activates PD-L1 promotes immunosuppression in breast cancer via the m6A/YTHDF3/PDK1 axis under hypoxic conditions
Introduction
Altered epigenetic reprogramming enables breast cancer cells to adapt to hypoxic stress. Hypoxic microenvironment can alter immune cell infiltration and function, limiting the effectiveness of immunotherapy.
Objectives
The study aimed to identify how fat mass and obesity-associated protein (FTO) helps breast cancer cells cope with the hypoxic microenvironment and the mechanisms behind breast cancer cell resistance to tumor immunity.
Methods
Clinical samples were utilized to analyze the impact of FTO on breast cancer progression and the effect of programmed cell death protein 1/ programmed cell death 1 ligand 1(PD-1/PD-L1) immune checkpoint inhibitor treatment. Utilized MeRIP-seq and mRNA-seq to analyze the downstream genes regulated by FTO under hypoxia. Methylation modification regulation of PDK1 by FTO was clarified using RIP. Then mouse models were utilized to analyze the efficacy of inhibiting FTO and 3-Phosphoinositide-dependent protein kinase 1(PDK1) in combination with PD-1/PD-L1 immune checkpoint inhibitor treatment.
Result
N6-Methyladenosine(m6A) demethylase FTO was transcriptionally activated by hypoxia inducible factor 1α(HIF-1α). PDK1 was identified as a potential target of FTO under hypoxic conditions through high-throughput sequencing. Mechanistically, overexpression of FTO decreases m6A modification sites on PDK1 mRNA, preventing YTH domain family 3(YTHDF3) from recognizing and binding to these sites, thereby inhibiting the degradation of PDK1 mRNA. Overexpression of PDK1 activates the AKT/STAT3 pathway, leading to enhanced PD-L1 expression. Targeting the FTO and PDK1-AKT pathways with FB23 and BX-912 inhibit breast cancer growth, enhance cytotoxic T lymphocyte (CTL) activity, and enhance the effectiveness of the PD-1/PD-L1 checkpoint inhibitor Atezolizumab.
Conclusion
This study reveals that HIF-1α promotes FTO transcription under hypoxic conditions, thereby increasing PD-L1 expression through the PDK1/AKT/STAT3 axis. Inhibition of FTO and PDK1 under hypoxic conditions could serve as a promising immunotherapeutic strategy for breast cancer.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


