肠大肠杆菌产生的耶尔希尼abactin促进克罗恩病中巨噬细胞的纤维化
IF 18.7
1区 医学
Q1 MICROBIOLOGY
引用次数: 0
摘要
炎症性肠病(IBD)相关纤维化会导致严重的发病率。其机制尚不清楚,但与微生物群,尤其是粘附侵袭性大肠杆菌(AIEC)有关。我们以前曾证实,在 IBD 小鼠模型中,产生金属噬菌体 yersiniabactin(Ybt)的 AIEC 会促进肠纤维化。由于巨噬细胞能解读微生物信号并影响炎症/组织重塑,我们推测 Ybt 金属螯合作用会破坏这一过程。在这里,我们发现巨噬细胞在人类 IBD 纤维化组织和小鼠纤维化病灶中大量存在,并与 AIEC 共定位。Ybt 通过低氧诱导因子 1-α(HIF-1α)(一种依赖金属的免疫调节因子)的稳定和核转位诱导巨噬细胞中组织坏死基因的表达。重要的是,产生 Ybt 的 AIEC 会消耗巨噬细胞内的锌,并通过抑制锌依赖的 HIF-1α 羟基化来稳定 HIF-1α。HIF-1α+巨噬细胞定位在人类IBD-纤维化狭窄和小鼠纤维化病变的疾病活动部位,突出了它们的生理相关性。我们的研究结果揭示了微生物群介导的金属螯合作用是一种针对炎症肠道中巨噬细胞的组织坏死触发器。本文章由计算机程序翻译,如有差异,请以英文原文为准。
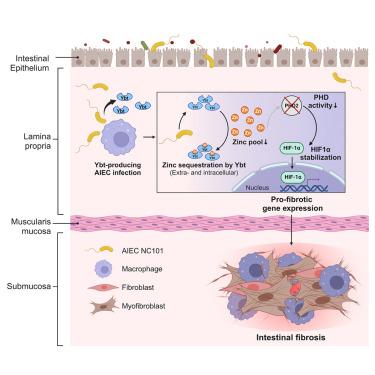
Intestinal E. coli-produced yersiniabactin promotes profibrotic macrophages in Crohn’s disease
Inflammatory bowel disease (IBD)-associated fibrosis causes significant morbidity. Mechanisms are poorly understood but implicate the microbiota, especially adherent-invasive Escherichia coli (AIEC). We previously demonstrated that AIEC producing the metallophore yersiniabactin (Ybt) promotes intestinal fibrosis in an IBD mouse model. Since macrophages interpret microbial signals and influence inflammation/tissue remodeling, we hypothesized that Ybt metal sequestration disrupts this process. Here, we show that macrophages are abundant in human IBD-fibrosis tissue and mouse fibrotic lesions, where they co-localize with AIEC. Ybt induces profibrotic gene expression in macrophages via stabilization and nuclear translocation of hypoxia-inducible factor 1-alpha (HIF-1α), a metal-dependent immune regulator. Importantly, Ybt-producing AIEC deplete macrophage intracellular zinc and stabilize HIF-1α through inhibition of zinc-dependent HIF-1α hydroxylation. HIF-1α+ macrophages localize to sites of disease activity in human IBD-fibrosis strictures and mouse fibrotic lesions, highlighting their physiological relevance. Our findings reveal microbiota-mediated metal sequestration as a profibrotic trigger targeting macrophages in the inflamed intestine.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell host & microbe
生物-微生物学
CiteScore
45.10
自引率
1.70%
发文量
201
审稿时长
4-8 weeks
期刊介绍:
Cell Host & Microbe is a scientific journal that was launched in March 2007. The journal aims to provide a platform for scientists to exchange ideas and concepts related to the study of microbes and their interaction with host organisms at a molecular, cellular, and immune level. It publishes novel findings on a wide range of microorganisms including bacteria, fungi, parasites, and viruses. The journal focuses on the interface between the microbe and its host, whether the host is a vertebrate, invertebrate, or plant, and whether the microbe is pathogenic, non-pathogenic, or commensal. The integrated study of microbes and their interactions with each other, their host, and the cellular environment they inhabit is a unifying theme of the journal. The published work in Cell Host & Microbe is expected to be of exceptional significance within its field and also of interest to researchers in other areas. In addition to primary research articles, the journal features expert analysis, commentary, and reviews on current topics of interest in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


