过渡金属氢化物键长与伸展波数的关系
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
这里证明了末端 3d 金属氢化物伸展波数 νMH 与迄今为止报告的金属氢化物距离 dMH 之间存在线性关系:νMH ∼ (-1.05dMH + 3.35) × 103 cm-1。也就是说,根据 X 射线衍射、分子光谱以及中子衍射的测定,当配合物和二元分子中的第 3 族金属移至第 10 族金属时,νMH(厘米-1)会随着金属氢距离 dMH(埃)的减小而增大。这一趋势与二磁性复合物相对恒定的键解离自由能(BDFE)和顺磁性复合物通常较小的 BDFE(可达 -20 kcal/mol)形成鲜明对比,从而表明 νMH 或其力常数与 BDFE 之间不存在相关性。如果通过金属离子氧化将正电荷添加到络合物中,νMH 可能会增加或减少,这取决于络合物的配位数和自旋状态。本文章由计算机程序翻译,如有差异,请以英文原文为准。
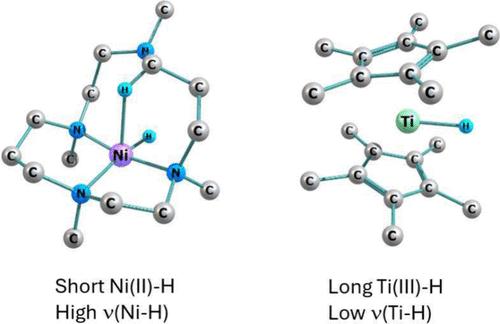
Relationship between Transition-Metal Hydride Bond Lengths and Stretching Wavenumbers
Here it is demonstrated that there is a linear relationship between the terminal 3d metal hydride stretching wavenumber νMH and the metal hydride distance dMH reported to date: νMH ∼ (−1.05dMH + 3.35) × 103 cm–1. That is, upon moving from Group 3 to Group 10 metals in complexes and binary molecules, νMH (cm–1) increases as the metal–hydrogen distance dMH (Å) decreases as determined by X-ray diffraction, molecular spectroscopy, and, in one case, neutron diffraction. This trend contrasts with the relatively constant bond dissociation free energy (BDFE) of the diamagnetic complexes and the usually smaller BDFE (by as much as −20 kcal/mol) of paramagnetic ones, thus showing that there is no correlation between either νMH or its force constant with the BDFE. If a positive charge is added to the complex by metal-ion oxidation, νMH may increase or decrease depending on the coordination number and spin state of the complex.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


