桥接1,2-二(二苯基膦)甲烷配体促进与二坐标和三坐标金离子形成双核配合物
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
五种新的结晶金(I)配合物 β-Au2(μ-dppm)2Br2-2CH2Cl2 (1)、[Au2(μ- dppm)2Br]Br-2CH2Cl2 (2)、[Au2(μ- dppm)2Br](PF6) (3)、(4) 和 [Au2(μ-dppm)2]Cl(AsF6)-2CH2Cl2 (5)(其中 dppm 为双(二苯基膦)甲烷),并通过单晶 X 射线衍射进行了结构表征。无色的 β-Au2(μ-dppm)2Br2-2CH2Cl2(1)具有中心对称结构,两个三配位金(I)离子被 dppm 配体紧紧包围。[Au2(μ-dppm)2Br]Br-2CH2Cl2(2)、[Au2(μ-dppm)2Br](PF6)(3)和[Au2(μ-dppm)2Cl](BPh4)-3CH2Cl2(4)晶体中的阳离子具有不寻常的排列方式,通过亲欧相互作用将二配位金(I)离子与三配位金(I)离子结合在一起。Au2(μ-dppm)2Cl(BPh4)-3CH2Cl2(4)中的氯离子只与复合物中的一个金离子结合,而[Au2(μ-dppm)2]Cl(AsF6)-2CH2Cl2(5)中的离子配对氯离子以相当长的距离对称地排列在两个金离子之间。在紫外线照射下,每个复合物都会发光。本文章由计算机程序翻译,如有差异,请以英文原文为准。
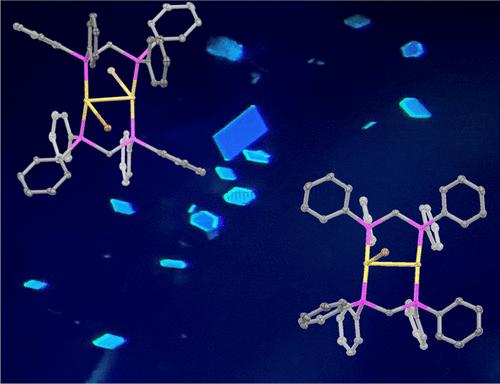
Bridging 1,2-Bis(diphenylphosphino)methane Ligands Facilitate the Formation of Binuclear Complexes with Both Two-Coordinate and Three-Coordinate Gold(I) Ions
Five new crystalline gold(I) complexes β-Au2(μ-dppm)2Br2·2CH2Cl2 (1), [Au2(μ- dppm)2Br]Br·2CH2Cl2 (2), [Au2(μ-dppm)2Br](PF6) (3), [Au2(μ-dppm)2Cl](BPh4)·3CH2Cl2 (4) and [Au2(μ-dppm)2]Cl(AsF6)·2CH2Cl2 (5) (where dppm is bis(diphenylphosphino)methane) have been prepared and structurally characterized by single crystal X-ray diffraction. Colorless β-Au2(μ-dppm)2Br2·2CH2Cl2 (1) has centrosymmetric structure with two three-coordinate gold(I) ions held in close proximity by the dppm ligands. Crystals of [Au2(μ- dppm)2Br]Br·2CH2Cl2 (2), [Au2(μ-dppm)2Br](PF6) (3), and [Au2(μ-dppm)2Cl](BPh4)·3CH2Cl2 (4) have a cation with an unusual arrangement that binds a two-coordinate gold(I) ion to a three-coordinate gold(I) ion through an aurophilic interaction. Whereas Au2(μ-dppm)2Cl(BPh4)·3CH2Cl2 (4) has a chloride ion bound to only one of the gold ions in the complex, [Au2(μ-dppm)2]Cl(AsF6)·2CH2Cl2 (5) has an ion paired chloride ion that is symmetrically disposed between the two gold ions at a rather long distance. Each complex displays luminescence under UV irradiation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


