IF 10.8
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
环境中的低剂量辐射(LDR)越来越普遍。然而,低剂量辐射暴露对免疫系统的影响仍然难以捉摸。在这里,我们有趣地发现,无论是在体外还是体内,低剂量辐射都能特异性地提高 CD4+IFNγ+ Th1 脾细胞的百分比,而不影响 CD8+IFNγ+ Tc1 细胞和调节性 T 细胞的百分比。在外周血 T 细胞中也发现了类似的现象。从机理上讲,我们发现 LDR 可诱导线粒体损伤,从而刺激 STING 信号通路,导致 Th1 细胞分化的主转录因子 T-bet 表达增强。特异性 STING 信号抑制剂能减弱 LDR 对 Th1 细胞分化的影响,证实了 STING 通路的核心作用。为了进一步验证LDR的免疫调节作用,我们让小鼠全身暴露于LDR,并评估LDR是否能通过增强抗肿瘤免疫来保护小鼠免受三阴性乳腺癌的侵袭。不出所料,LDR 明显延缓了肿瘤的发展并促进了细胞的死亡。同时,LDR 增加了肿瘤浸润的 Th1 细胞,而 Tc1 和 Treg 细胞的比例保持不变。此外,抗肿瘤巨噬细胞的浸润也增加了。总之,我们发现环境 LDR 可以特异性调节 Th1 T 细胞的活性,这为 LDR 在临床和非临床环境中的潜在应用提供了关键信息。本文章由计算机程序翻译,如有差异,请以英文原文为准。
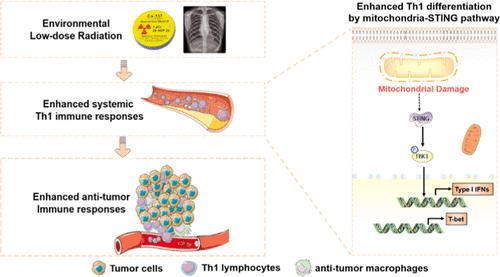
Environmental Low-Dose Radiation Activates Th1 Immunity through the Mitochondria-STING Pathway
The presence of low-dose radiation (LDR) in the environment has become more prevalent. However, the effect of LDR exposure on the immune system remains elusive. Here, we interestingly found that LDR specifically elevated the percentage of CD4+IFNγ+ Th1 splenocytes, both in vitro and in vivo, without affecting the percentage of CD8+IFNγ+ Tc1 cells and regulatory T cells. A similar phenomenon was found in T cells from peripheral blood. Mechanistically, we found that LDR can induce mitochondrial damage, which stimulated the STING signaling pathway, leading to the enhanced expression of T-bet, the master transcriptional factor of Th1-cell differentiation. The specific STING signal inhibitor can abrogate the effect of LDR on Th1 differentiation, confirming the central role of the STING pathway. To further validate the immunoregulatory role of LDR, we exposed mice with whole body LDR and evaluated if LDR could protect mice against triple-negative breast cancer through enhanced antitumor immunity. As expected, LDR significantly delayed tumor development and promoted cell death. Meanwhile, LDR resulted in increased tumor-infiltrating Th1 cells, while the proportion of Tc1 and Treg cells remained unchanged. Furthermore, the infiltration of antitumor macrophages was also increased. In summary, we revealed that environmental LDR could specifically regulate Th1 T-cell activities, providing critical information for the potential application of LDR in both clinical and nonclinical settings.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


