藻类胞外有机物介导氧化锰还原溶解机理及对17α-炔雌醇去除的影响
IF 10.8
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
氧化锰(MnOx)的还原溶解是提高自然水生环境中锰可用性的一个主要过程。富营养化水体中普遍存在的藻类分泌的胞外有机物(EOM)可能会影响氧化锰的溶解,从而影响有机微污染物的归宿。本研究探讨了 EOM 介导的氧化锰还原溶解机制,并研究了这一过程对 17α-ethinylestradiol 降解的影响。研究评估了在黑暗和辐照条件下 EOM 浓度(1.0-20.0 mgC/L)和 pH 值(6.0-9.0)的影响。研究发现,在黑暗条件下,EOM 可通过配体-金属电荷转移(LMCT)促进氧化锰的还原溶解。在辐照条件下,超氧离子(O2--)的参与进一步促进了溶解。EOM浓度越高,可利用的还原物质和O2--的含量就越高,从而加速了还原溶解。较高的 pH 值减缓了光还原溶解速度,而 O2--介导的还原作用变得更加重要。研究发现,EOM 中的多酚和高度不饱和碳及酚类物质是还原溶解的驱动力。氧化锰还原溶解形成的可溶性活性锰(III)可有效去除溶液中的17α-炔雌醇。总之,有关氧化锰还原溶解机制的研究结果对锰的地球化学循环和有机微污染物的归宿具有广泛的影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
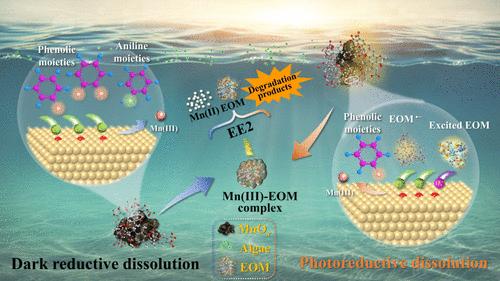
Reductive Dissolution Mechanisms of Manganese Oxide Mediated by Algal Extracellular Organic Matter and the Effects on 17α-Ethinylestradiol Removal
Reductive dissolution of manganese oxide (MnOx) is a major process that improves the availability of manganese in natural aquatic environments. The extracellular organic matter (EOM) secreted by algae omnipresent in eutrophic waters may affect MnOx dissolution thus the fate of organic micropollutants. This study investigates the mechanisms of MnOx reductive dissolution mediated by EOM and examines the effects of this process on 17α-ethinylestradiol degradation. The influences of EOM concentration (1.0–20.0 mgC/L) and pH (6.0–9.0) in both dark and irradiated conditions were assessed. In the dark, EOM was found to facilitate MnOx reductive dissolution via the ligand-to-metal charge transfer (LMCT). The dissolution was further enhanced under irradiation, with the participation of superoxide ions (O2•–). Higher EOM concentrations increased the contents of available reducing substances and O2•–, accelerating the reductive dissolution. Higher pH slowed the photoreductive dissolution rates, while O2•–-mediated reduction became more important. Polyphenols and highly unsaturated carbon and phenolic formulas in EOM were found to drive the reductive dissolution. Soluble reactive Mn(III) formed through reductive dissolution of MnOx effectively removed 17α-ethinylestradiol in solution. Overall, the findings regarding the mechanisms behind reductive dissolution of MnOx have broad implications for Mn geochemical cycles and organic micropollutant fate.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


