IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
被称为氧进化反应(OER)的阳极过程的缓慢动力学给质子交换膜水电解槽在工业环境中的实际应用带来了巨大挑战。本研究通过两步燃烧法将氧化铱纳米颗粒(IrO2)锚定在氧化钴(Co3O4)基底上,从而引入了一种高性能 OER 催化剂。由此制备的 IrO2@Co3O4 催化剂在酸性环境中的催化活性和稳定性都有显著提高。值得注意的是,达到 10 mA cm-2 电流密度(常用的比较基准)所需的过电位仅为 301 mV。此外,过电位的升高幅度极小,这也证实了它在 80 小时的持续时间内都能保持稳定。能谱表征和实验结果表明,IrO2@Co3O4 表面 OER 活性 Ir3+ 物种的生成是由 IrO2 和 Co3O4 之间的强相互作用引起的。理论计算进一步表明,负载在 Co3O4 上的 IrO2 位点在 *OOH 去质子化形成解吸 O2 时具有较低的能障。此外,这种相互作用还能保持铱活性位点的化学状态,从而稳定铱活性位点,提高其长期稳定性。这些见解可能会对设计和合成更高效的 OER 电催化剂的战略产生重大影响,使其在工业中得到更广泛的应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
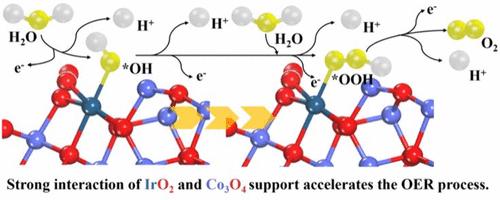
Enhanced Acidic Oxygen Evolution Reaction Performance by Anchoring Iridium Oxide Nanoparticles on Co3O4
The sluggish kinetics of the anodic process, known as the oxygen evolution reaction (OER), has posed a significant challenge for the practical application of proton exchange membrane water electrolyzers in industrial settings. This study introduces a high-performance OER catalyst by anchoring iridium oxide nanoparticles (IrO2) onto a cobalt oxide (Co3O4) substrate via a two-step combustion method. The resulting IrO2@Co3O4 catalyst demonstrates a significant enhancement in both catalytic activity and stability in acidic environments. Notably, the overpotential required to attain a current density of 10 mA cm–2, a commonly used benchmark for comparison, is merely 301 mV. Furthermore, stability is maintained over a duration of 80 h, as confirmed by the minimal rise in overpotential. Energy spectrum characterizations and experimental results reveal that the generation of OER-active Ir3+ species on the IrO2@Co3O4 surface is induced by the strong interaction between IrO2 and Co3O4. Theoretical calculations further indicate that IrO2 sites loaded onto Co3O4 have a lower energy barrier for *OOH deprotonation to form desorbed O2. Moreover, this interaction also stabilizes the iridium active sites by maintaining their chemical state, leading to superior long-term stability. These insights could significantly impact the strategies for designing and synthesizing more efficient OER electrocatalysts for broader industrial application.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


