溶解度等温线的乘法性
IF 3.8
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
在等温线方程的帮助下,溶解度随增溶剂浓度的变化("溶解度等温线")可以揭示潜在的相互作用。然而,尽管这些简单的统计热力学等温线方程成功地阐明了水合作用(通过合作(sigmoidal)等温线)和协同溶解作用(通过二次(钟形)等温线)的机理,但对于结合了它们特征的更复杂的等温线来说,仅靠这些方程是不够的。在此,我们将展示:(i) 简单等温线如何通过建立在过剩数关系(即溶质周围增溶剂过剩数的增溶剂浓度依赖性)基础上的等温线乘法法则进行组合;(ii) (i) 如何成功模拟复杂的溶解度等温线,捕捉到溶质与增溶剂之间的合作关系反过来又会导致增溶剂在较高浓度下被排斥出已经拥挤不堪的溶质区域。此外,我们还将证明合作溶解度等温线和二次溶解度等温线都可以直接从过剩数量关系中推导出来,从而使其不仅成为乘法规则的基础,而且成为简单和复杂溶解度等温线的基本关系。本文章由计算机程序翻译,如有差异,请以英文原文为准。
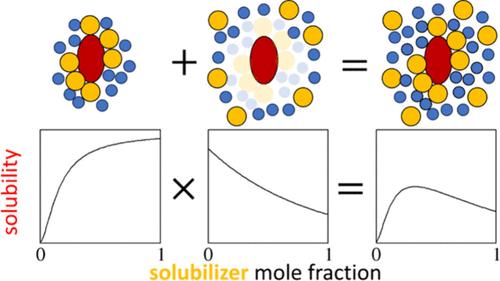
Multiplicativity in Solubility Isotherms
With the help of isotherm equations, how solubility changes with solubilizer concentration (“solubility isotherm”) can reveal the underlying interactions. However, despite their success in elucidating the mechanisms of hydrotropy (via the cooperative (sigmoidal) isotherm) and synergistic solvation (via the quadratic (bell-shaped) isotherm), these simple statistical thermodynamic isotherm equations alone are insufficient for more complex isotherms that combine their features. Here, we show (i) how simple isotherms can be combined via the isotherm multiplicativity rule founded on the excess number relationship (i.e., solubilizer concentration dependence of the solubilizer excess number around a solute) and (ii) how (i) leads to successful modeling of complex solubility isotherms, capturing that cooperative solute–solubilizer association, in turn, induces the exclusion of solubilizers from the already crowded solute’s locality at higher concentrations. Moreover, we will demonstrate that both the cooperative and quadratic solubility isotherms can be derived directly from the excess number relationship, establishing it not only as the basis for the multiplicativity rule but also as the fundamental relationship for simple and complex solubility isotherms.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


