酶法形成氮-氮键的最新进展与挑战
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
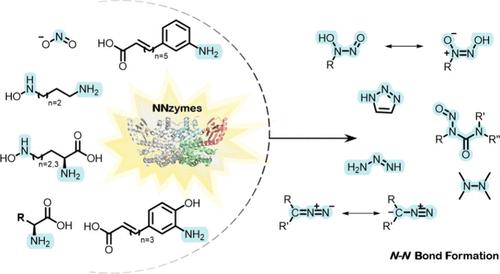
Recent Developments and Challenges in the Enzymatic Formation of Nitrogen–Nitrogen Bonds
The biological formation of nitrogen–nitrogen (N–N) bonds represents intriguing reactions that have attracted much attention in the past decade. This interest has led to an increasing number of N–N bond-containing natural products (NPs) and related enzymes that catalyze their formation (referred to in this review as NNzymes) being elucidated and studied in greater detail. While more detailed information on the biosynthesis of N–N bond-containing NPs, which has only become available in recent years, provides an unprecedented source of biosynthetic enzymes, their potential for biocatalytic applications has been minimally explored. With this review, we aim not only to provide a comprehensive overview of both characterized NNzymes and hypothetical biocatalysts with putative N–N bond forming activity, but also to highlight the potential of NNzymes from a biocatalytic perspective. We also present and compare conventional synthetic approaches to linear and cyclic hydrazines, hydrazides, diazo- and nitroso-groups, triazenes, and triazoles to allow comparison with enzymatic routes via NNzymes to these N–N bond-containing functional groups. Moreover, the biosynthetic pathways as well as the diversity and reaction mechanisms of NNzymes are presented according to the direct functional groups currently accessible to these enzymes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


