揭示酒精对高价碘(III)催化的不对称酚类脱芳烃反应的添加效应:配体取代和低阻氢键
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
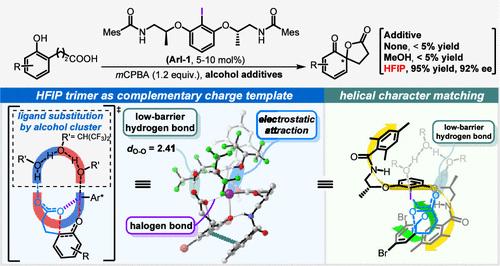
Unraveling Alcohol Additive Effects on Hypervalent Iodine(III)-Catalyzed Asymmetric Phenolic Dearomatization: Ligand Substitution and Low-Barrier Hydrogen Bonds
Despite the widespread use of hexafluoropropanol (HFIP) as a “magic” solvent or additive in organic synthesis, its fundamental mechanisms lag far behind. This study presents mechanistic insights into the puzzling alcohol additive effects observed in Ishihara’s conformationally flexible C2-symmetric iodoarene-catalyzed asymmetric phenolic dearomatization through density functional theory calculations. The results reveal that due to the “booster effect” of fluorinated alcohols, HFIP assembles a trimeric hydrogen bond cluster that displaces a ligand from the active iodine(III) catalyst and forms a low-barrier hydrogen bond with the substrate, which significantly enhances the oxidizing power of the iodine(III) center, thus facilitating the dearomatization of electron-deficient phenols. Conversely, methanol is found to promote the dearomatization of electron-rich phenols via a formally similar yet distinct mechanism, thus highlighting the unique role of HFIP as an additive. The insights gained from this investigation advance our molecular-level understanding of the synergistic interactions between catalysts and additives, potentially guiding the design of catalytic systems that exploit these effects for broader applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


