通过氮置换在 M0.5Co0.5O 中构建富电子金属位,以高效激活过一硫酸盐,从而降解有机污染物
IF 9.7
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
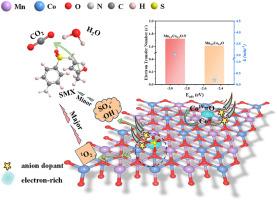
Constructing electron-rich metal sites in M0.5Co0.5O through N substitution for efficient peroxymonosulfate activation to degrade organic pollutants
Transition metal oxides are promising heterogeneous catalysts for peroxymonosulfate (PMS) activation. However, the catalytic degradation performance was unsatisfactory. Herein, nitrogen doping was applied to construct electron-rich metal sites in bimetallic oxides (Mn0.5Co0.5O, Fe0.5Co0.5O, Cu0.5Co0.5O) to boost their PMS activation performance for sulfamethoxazole (SMX) degradation. The N-doped bimetallic oxides (Mn0.5Co0.5O-N, Fe0.5Co0.5O-N, Cu0.5Co0.5O-N), obtaining through a facile ammonia-assisted medium-temperature heat treatment method, displayed enhanced PMS activation performance for SMX degradation compared with the pristine bimetallic oxides. Especially, Mn0.5Co0.5O-N is the optimal option with 100% SMX degradation efficiency within 2 min, wide pH application range (3.5-11.5), and excellent cycling performance. The density functional theory (DFT) calculations confirmed that Mn0.5Co0.5O-N with more negative adsorption energy (Eads) and higher electron transfer number was more beneficial for PMS adsorption and activation. Quenching experiments, electron paramagnetic resonance (EPR), and solvent exchange (H2O to D2O) indicated that 1O2 contributed predominantly to SMX degradation. This research offers an economical strategy for boosting the PMS activation activity to degrade pollutants of transition metal oxides through constructing electron-rich metal sites in bimetallic oxides by N substitution.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Cleaner Production
环境科学-工程:环境
CiteScore
20.40
自引率
9.00%
发文量
4720
审稿时长
111 days
期刊介绍:
The Journal of Cleaner Production is an international, transdisciplinary journal that addresses and discusses theoretical and practical Cleaner Production, Environmental, and Sustainability issues. It aims to help societies become more sustainable by focusing on the concept of 'Cleaner Production', which aims at preventing waste production and increasing efficiencies in energy, water, resources, and human capital use. The journal serves as a platform for corporations, governments, education institutions, regions, and societies to engage in discussions and research related to Cleaner Production, environmental, and sustainability practices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


