Cl-对CeO2-WO3/TiO2催化剂NH3选择性催化还原NO的影响
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
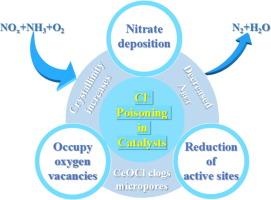
本文利用具有成本效益的氯化铈代替传统的硝酸铈作为铈源,制备了 30CeO2-4WO3/TiO2 催化剂。对催化剂的结构、理化性质和催化性能进行了表征。随着 Cl- 含量的增加,30CeO2-4WO3/TiO2 催化剂的催化性能和 N2 选择性明显下降。具体来说,30CeO2-4WO3/TiO2-0.007 % 的脱硝效率超过 80 % 和 90 %,分别为 225 ∼ 520 ℃ 和 241 ∼ 504 ℃,而 30CeO2-4WO3/TiO2-0.161 % 样品的最高脱硝效率仅为 79.83 %。讨论了 Cl- 对催化性能的影响机理。(1) XRD 和 BET 结果表明,Cl- 能使 TiO2 和 CeO2 的晶格膨胀,从而增加其结晶度并降低比表面积。(2)Cl- 与 NH4+ 作用生成 NH4Cl,然后与 CeO2 反应生成 CeOCl,导致催化剂微孔阻塞。(3) XPS、NH3-TPD 和 H2-TPR 分析表明,Cl- 会占据氧空位,阻碍氧迁移并减少活性表面中心。(4) 原位 DRIFTs 分析表明,Cl- 浓度升高会降低 NH3 的吸附能力,促进硝酸盐的形成。硝酸盐的积累是催化剂活性下降的主要原因。本文章由计算机程序翻译,如有差异,请以英文原文为准。

The effect of Cl- on CeO2-WO3/TiO2 catalyst for selective catalytic reduction of NO with NH3
The 30CeO2-4WO3/TiO2 catalyst was prepared utilizing cost-effective cerium chloride instead of the traditional cerium nitrate as the cerium source in this paper. The structure, physical and chemical properties, catalytic performance were characterized. The catalytic performance and N2 selectivity of the 30CeO2-4WO3/TiO2 catalyst significantly deteriorate with increased the content of Cl-. Specifically, denitration efficiency of 30CeO2-4WO3/TiO2-0.007 % exceeding 80 % and 90 % were 225 ∼ 520 °C and 241 ∼ 504 °C, respectively, whereas the maximum denitration efficiency of the 30CeO2-4WO3/TiO2-0.161 % sample was only 79.83 %. The effect mechanism of Cl- on catalytic performance was discussed. (1) XRD and BET results show that Cl- can cause the expansion of the lattice of TiO2 and CeO2, thereby increasing its crystallinity and decreasing the specific surface area. (2) Cl- interacts with NH4+ to form NH4Cl, which subsequently reacts with CeO2 to produce CeOCl, leading to the obstruction of the catalyst’s micropores. (3) XPS, NH3-TPD, and H2-TPR analyses reveal that Cl- would occupy the oxygen vacancies, impeding oxygen migration and diminishing active surface centers. (4) In-situ DRIFTs analysis demonstrates that elevated Cl- concentrations diminish NH3 adsorption capacity and facilitate nitrate species formation. The accumulation of nitrates was a primary cause of the decline in catalyst activity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


