电气石/碳负载Cu-Ni/ZrO2活化水相系统高效催化乙酰丙酸加氢的快速构建
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
乙酰丙酸(LA)高效加氢制备γ-戊内酯(GVL)反应体系的激活对生物质的可持续发展具有重要意义。本文以电气石(TM)活化的水相体系为策略,采用全固相法制备了Cu-Ni/ZrO2@TM/C催化剂。Cu-Ni/ZrO2@TM/C表现出优异的催化性能,这得益于电气石的自发极化和远红外辐射特性以及金属-金属和金属-载体的强相互作用。在180 °C、150 min、1.5 MPa H2条件下,LA转化率为100.0% %,GVL收率为93.0 %。各种实验和理论计算证实,电气石具有自发极化电场和远红外辐射,可以打破分子间氢键,形成小的水团,激活水相环境,促进活性物质的运输,并大大降低氢化屏障。电气石的活化水效应、ZrO2的酸位和氧空位以及Cu-Ni双金属的合金结构可以协同促进活性物质的输运,增强水辅助质子跳跃。此外,还提出了一种合理的电气石活化水相体系来解释LA水加氢的增强。本研究创新性地阐明了生物质衍生化学品高效水相加氢功能非贵金属催化剂的构建。本文章由计算机程序翻译,如有差异,请以英文原文为准。
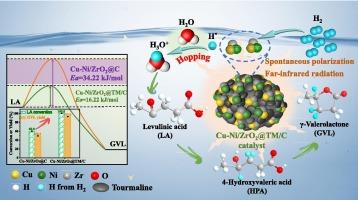
Facile construction of tourmaline/carbon-supported Cu-Ni/ZrO2 for efficient catalytic hydrogenation of levulinic acid via activating aqueous-phase system
The activation of reaction system for high-efficiency hydrogenation of levulinic acid (LA) into γ-valerolactone (GVL) is significant for sustainable development of biomass. Herein, based on a strategy of tourmaline (TM)-activated aqueous-phase system, a Cu-Ni/ZrO2@TM/C catalyst was facilely constructed by a totally solid-phase method. Cu-Ni/ZrO2@TM/C exhibited outstanding catalytic performance, benefiting from the spontaneous polarization and far-infrared radiation characteristics of tourmaline and the strong metal–metal and metal–support interactions. Under the optimum reaction conditions of 180 °C, 150 min, and 1.5 MPa H2, 100.0 % of LA conversion and 93.0 % of GVL yield were achieved. A variety of tests and theoretical calculations confirm that tourmaline with spontaneously polarized electric field and far-infrared radiation can break intermolecular hydrogen bonds to form small water clusters for activating aqueous-phase environment, facilitating the transport of active species as well as greatly reducing the hydrogenation barrier. The activating water effect of tourmaline, the acid sites and oxygen vacancies of ZrO2, and the alloying structure of Cu-Ni bimetal can synergistically benefit the transport of active species for reinforcing water-assisted proton hopping. Moreover, a reasonable tourmaline-activated aqueous-phase system was proposed to explain the enhanced aqueous hydrogenation of LA. This study innovatively illuminates the construction of functional non-precious metal catalysts for efficient aqueous-phase hydrogenation of biomass-derived chemicals.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


