多功能电解质添加剂实现了稳定锌金属阳极的适应性界面
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
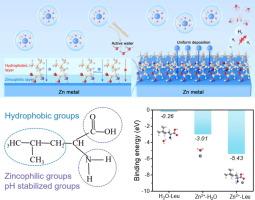
H2 演化、锌枝晶形成和副反应严重阻碍了锌离子水电池的实际应用。在此,一种多功能亮氨酸添加剂被引入到 ZnSO4 电解液中,以创建适应性界面。极性基团使亮氨酸优先吸附在锌金属表面,诱导 Zn2+ 均匀沉积,抑制锌枝晶的生长,而疏水基团则阻止锌金属与水接触,抑制与水相关的副反应。同时,齐聚氨基和羧基段可以动态调节 pH 值的变化,形成稳定的界面微环境,从而抑制副反应。此外,亮氨酸对 Zn2+ 的高亲和力调节了溶解结构,有利于在金属锌上形成无机 ZnS 层,降低 Zn 离子的解溶解能垒,提高 Zn 离子的电镀动力学。作为概念验证,与裸 ZnSO4 电解质(200 小时)相比,含有亮氨酸添加剂的对称电池在 1.0 mA cm-2/1.0 mAh cm-2 条件下的循环寿命延长了 2700 小时。此外,与 VO2 阴极配对的全电池显示出卓越的长期循环稳定性,1800 次循环后容量保持率达 91%,可作为灵活的储能系统为电子表供电。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Multifunctional electrolyte additives enabled adaptable interface toward stabilizing Zn metal anodes
H2 evolution, Zn dendrites formation, and side reactions have severely hindered the practical applications of aqueous zinc-ion batteries. Herein, a multifunctional leucine additive is introduced into ZnSO4 electrolyte to create adaptable interfaces. The polar groups make leucine preferentially adsorb on Zn metal surface, inducing Zn2+ uniform deposition to suppress Zn dendrites growth, while the hydrophobic groups prevent Zn metal contacting from water, inhibiting water-related side reactions. Meanwhile, the zwitterionic amino and carboxyl segments can dynamically adjust the pH changes with a stable interfacial microenvironment, thereby inhibiting side reactions. Besides, the high affinity of leucine to Zn2+ regulated the solvated structure and facilitated forming inorganic ZnS layer on Zn metal, reducing the Zn-ions desolvation energy barrier and enhancing Zn-ions plating kinetics. As a proof of concept, the symmetric cells with leucine additives exhibit an extended cycling lifetime of 2700 h at 1.0 mA cm−2/1.0 mAh cm−2 when compared to bare ZnSO4 electrolyte (200 h). Moreover, the full cells pairing with VO2 cathodes displayed superior long-term cycling stability with 91 % capacity retention after 1800 cycles, which can work as flexible energy storage systems to power electronic watch.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


