钠键是定向的吗?
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
对四配位和五配位的钠配合物进行了统计分析和计算研究。虽然没有几何校正的统计分析表明,大多数的几何结构远非线性,但经几何校正的分析表明,这些键是有方向性和线性的。计算研究表明,粘结强度可以达到中等强度。本文章由计算机程序翻译,如有差异,请以英文原文为准。
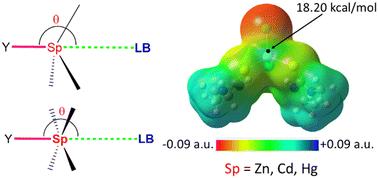
Are spodium bonds directional?†
Statistical analyses and computational studies have been performed on spodium bonds with four- and five-coordinated spodium complexes. Though statistical analyses without geometry correction suggest that the most populous geometries are far from linearity, geometry-corrected analyses suggest that these bonds are directional and linear. Computational studies suggest that the bond strength can be moderately strong.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


