使用数字双 CRISPR-Cas 检测法在单个细胞外囊泡水平同时检测蛋白质和 miRNA
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
由于肿瘤来源的细胞外囊泡的异质性和稀缺性,在单个细胞外囊泡(EV)水平上同时检测蛋白质和microRNA (miRNA)显示出精确的疾病谱分析的巨大希望。然而,一种高可靠的单辆电动汽车多靶点分析方法仍有待开发。本研究提出了一种数字双crispr - cas驱动的单EV评估(ddSEE)系统,可以在单分子水平上同时检测EV的表面蛋白和内部miRNA。通过优化CRISPR-Cas12a和CRISPR-Cas13a的同时反应条件,Cas12a利用抗体-DNA偶联物将蛋白信号传递至DNA,检测ev表面蛋白,Cas13a通过ev -脂质体融合分析ev内部miRNA。利用含有188,000个微孔的微流控芯片将CRISPR-Cas系统转化为数字分析格式,通过荧光成像无偏置地绝对定量miRNA/protein阳性ev,最低可检测214 ev /μL。最后,总共收集和测试了31份血液样本,其中21份来自乳腺癌患者,10份来自健康献血者,在区分乳腺癌患者和健康献血者方面的诊断准确率达到92%。凭借其绝对定量、易用性和多路检测能力,ddSEE系统在EV研究和临床应用中都显示出巨大的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
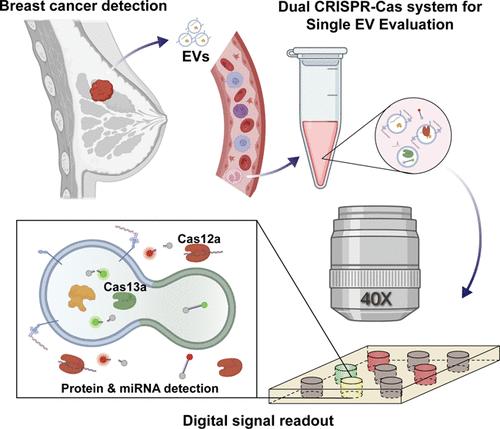
Concurrent Detection of Protein and miRNA at the Single Extracellular Vesicle Level Using a Digital Dual CRISPR-Cas Assay
The simultaneous detection of proteins and microRNA (miRNA) at the single extracellular vesicle (EV) level shows great promise for precise disease profiling, owing to the heterogeneity and scarcity of tumor-derived EVs. However, a highly reliable method for multiple-target analysis of single EVs remains to be developed. In this study, a digital dual CRISPR-Cas-powered Single EV Evaluation (ddSEE) system was proposed to enable the concurrent detection of surface protein and inner miRNA of EVs at the single-molecule level. By optimizing simultaneous reaction conditions of CRISPR-Cas12a and CRISPR-Cas13a, the surface protein of EVs was detected by Cas12a using antibody-DNA conjugates to transfer the signal of the protein to DNA, while the inner miRNA was analyzed by Cas13a through EV-liposome fusion. A microfluidic chip containing 188,000 microwells was used to convert the CRISPR-Cas system into a digital assay format to enable the absolute quantification of miRNA/protein-positive EVs without bias through fluorescence imaging, which can detect as few as 214 EVs/μL. Finally, a total of 31 blood samples, 21 from breast cancer patients and 10 from healthy donors, were collected and tested, achieving a diagnostic accuracy of 92% in distinguishing patients with breast cancer from healthy donors. With its absolute quantification, ease of use, and multiplexed detection capability, the ddSEE system demonstrates its great potential for both EV research and clinical applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


