利用胺-酸偶联反应在系统水平上调节 BRD4 PROTAC 的效力
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
利用蛋白水解靶向嵌合体(PROTACs)降解蛋白质是一种前景广阔的治疗策略。PROTACs 是一种杂多功能分子,由目标结合分子和 E3 连接酶结合分子组成,并通过连接体连接。这些片段通常通过酰胺键结合在一起。虽然酰胺可以直接合成,但可能会给整个分子带来不理想的药物特性。从系统的角度来看,我们设想可以通过选择反应条件来调节 PROTAC 的药效--在不同的催化剂下,相同的两个结构单元会产生不同的连接体。除了经典的酰胺偶联反应外,我们还通过四种新的胺酸偶联反应制备了一套 BRD4 PROTAC 降解剂。我们的研究结果表明,反应条件的变化会影响 PROTAC 的理化性质,从而产生一系列不同的性质。值得注意的是,与采用酰胺连接物的PROTACs相比,几种新的PROTACs显示出更强的BRD4降解功效,这强调了系统化学作为一种治疗优化策略的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
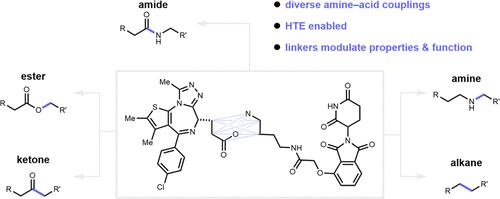
Modulating the Potency of BRD4 PROTACs at the Systems Level with Amine-Acid Coupling Reactions
Protein degradation using proteolysis targeting chimeras (PROTACs) represents a promising therapeutic strategy. PROTACs are heterobifunctional molecules that consist of a target-binding moiety and an E3 ligase binding moiety, connected by a linker. These fragments are frequently united via amide bonds. While straightforward to synthesize, amides may impart suboptimal drug properties to the overall molecule. From a systems level perspective, we envisioned that the potency of PROTACs could be modulated through selection of reaction conditions─wherein different catalysts produce distinct linkers from the same two building blocks. We present a suite of BRD4 PROTAC degraders prepared via four new amine–acid coupling reactions alongside the classic amide coupling. Our findings reveal that variations in reaction conditions affect the physicochemical properties of PROTACs, resulting in a spectrum of properties. Notably, several new PROTACs demonstrated enhanced BRD4 degradation efficacy compared to those employing amide linkers, emphasizing the potential of systems chemistry as a therapeutic optimization strategy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


