调节框架外阳离子对LTA沸石中银团簇发光性能的影响及理论建模
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
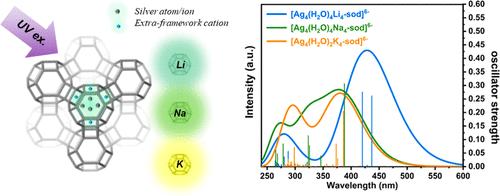
多孔沸石内的发光银簇由于其高量子产率、发射可调、优异的化学稳定性和良好的采用能力等优点而引起了广泛的研究兴趣。本研究合成了一系列Ag交换的R- lta /Ag (R = Li, Na, K)分子筛,其中银团簇在紫外区激发蓝移,可见光区发射红移,分别以Li+、Na+和K+为框架外阳离子时发射强度降低。[Ag4(H2O)xR4-sod]6 - (x = 2,4;构建了R = Li, Na, K)结构,并进一步采用TD-DFT方法研究了框架外阳离子对[Ag4]2+簇发光性能的影响,其中考虑了Al取代Si和合理的水合水平。估计[Ag4(H2O)4Li4-sod]6 -簇的S1和S3能级、[Ag4(H2O)4Na4-sod]6 -簇的S4能级和[Ag4(H2O)2K4-sod]6 -簇的S5能级可以通过直接激发有效填充,并假设S1→S0跃迁是[Ag4]2+假四面体可见光区辐射发射的原因。[Ag4(H2O)xR4-sod]6 - (x = 2,4;R = Li, Na, K)团簇解释了本研究和文献报道的R- lta /Ag (R = Li, Na, K)沸石中银团簇的修饰发光特性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Manipulation and Theoretical Modeling of the Luminescence Performance of Silver Clusters in LTA Zeolites by Adjusting Extra-framework Cations
Luminescent silver clusters that are confined inside porous zeolites have attracted great research interest due to the combined advantages of high quantum yield, tunable emission, excellent chemical stability, and desirable adoption ability of this micronano-composite. In this research, a series of Ag-exchanged R-LTA/Ag (R = Li, Na, K) zeolites were synthesized, in which the silver clusters showed blue-shifted excitation in the UV region, red-shifted emission in the visible region, and decreased emission intensity when taking Li+, Na+, and K+ as the extra-framework cations, respectively. The [Ag4(H2O)xR4-sod]6– (x = 2, 4; R = Li, Na, K) structures were constructed, and the TD-DFT method was further carried out to study the influence of extra-framework cations on the luminescence performance of [Ag4]2+ clusters, in which the substitution of Al for Si and the reasonable hydrate levels were both taken into consideration. It is estimated that the S1 and S3 levels for the [Ag4(H2O)4Li4-sod]6– cluster, the S4 level for the [Ag4(H2O)4Na4-sod]6– cluster, and the S5 level for the [Ag4(H2O)2K4-sod]6– cluster can be effectively populated through direct excitation, and the S1 → S0 transition is assumed to be responsible for the radiative emission in the visible region for the [Ag4]2+ pseudo-tetrahedrons. This calculated energy level diagram and simulated absorption spectra of [Ag4(H2O)xR4-sod]6– (x = 2, 4; R = Li, Na, K) clusters explained the modified luminescence property of silver clusters in R-LTA/Ag (R = Li, Na, K) zeolites studied in this research and reported literature.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


