磷酸盐与偕胺肟协同回收海水中铀的理论探索
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
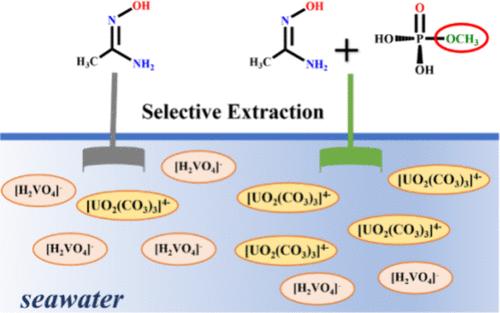
目前,从海水中高选择性地提取铀极具挑战性。虽然脒肟基团(HAO)是海水铀萃取中常用的配体,但它对钒离子也有很强的结合能力。有研究表明,在脒肟基吸附剂中引入磷酸基团可以通过官能团之间的协同效应提高材料的吸附性能。在这项工作中,我们利用密度泛函理论(DFT)系统地研究了磷酸配体(甲基膦酸,HL1)对铀阳离子的选择性萃取行为以及与脒肟的协同效应。研究还考虑了 HL1 的电子供体取代衍生物(氨甲基膦酸 (HL2) 和磷酸甲酯 (HL3))。结果不出所料,在脒肟基吸附剂中引入 HL1 可以提高铀阳离子的萃取率和选择性。这主要是由于 HL1 更容易去质子化,从而促进 [UO2(CO3)3]4- 的解离,而且协同复合物中磷酸基团的存在改变了 VO2+ 的最佳配位模式。此外,HL2 和 HL3 配体进一步提高了铀萃取性能,尤其是 HL3。这项工作为合理设计用于从海水中提取铀的固着配体提供了指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Theoretical Exploration of the Synergistic Effect of Phosphate and Amidoxime for Uranium Recovery from Seawater
Highly selective extraction of uranium from seawater is currently extremely challenging. Although the amidoxime group (HAO) is the commonly used ligand in seawater uranium extraction, it also has strong binding capacity for vanadium ion. It has been shown that the introduction of phosphate groups into amidoxime-based adsorbents can improve the adsorption performance of materials through a synergistic effect between functional groups. In this work, we have systematically investigated the selective extraction behavior of the phosphate ligand (methylphosphonic acid, HL1) for uranyl cation and the synergistic effect with amidoxime using density functional theory (DFT). The electron-donor-substituted derivatives of HL1 (aminomethylphosphonic acid (HL2) and methyl phosphate (HL3)) were also considered. Not unexpectedly, the results show that introduction of HL1 into the amidoxime-based adsorbents improves the extraction and selectivity for uranyl cations. This is mainly due to the fact that HL1 is more likely to deprotonate, which promotes the dissociation of [UO2(CO3)3]4–, and the presence of the phosphate groups in the synergistic complexes alters the optimal coordination mode of VO2+. In addition, the HL2 and HL3 ligands further improve the uranium extraction performance, especially for HL3. This work provides guidelines for the rational design of sequestering ligands for uranium extraction from seawater.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


