手性三唑类杀菌剂伊康唑在土壤和蚯蚓中的对映体分离、生物活性、环境命运和毒性
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
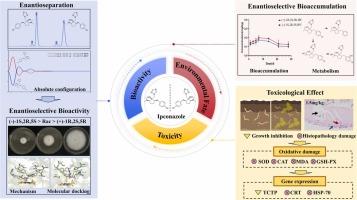
异康唑(IPC)是一种手性三唑类杀菌剂,常用于种子病害防治。本研究从手性角度研究了异康唑对病原微生物的生物活性和潜在机制。研究还探讨了异康唑对映体在土壤-蚯蚓系统中的积累行为,并评估了其对蚯蚓的毒性作用。生物活性评价发现,异康唑对三种植物病原菌的生物活性顺序为(-)-1S,2 R,5S-IPC>rac-IPC>(+)-1R,2S,5R-IPC,其中(-)-1S,2 R,5S-IPC的生物活性是(+)-1R,2S,5R-IPC的34.6-129.5倍。分子对接发现,(-)-1S,2 R,5S-IPC 与靶蛋白 CYP51 的结合亲和力更强,从而导致活性差异。积累和代谢研究发现,(-)-1S,2 R,5S-IPC比(+)-1R,2S,5R-IPC更持久,异康唑在土壤-蚯蚓系统中主要通过羟化作用代谢为羟基异康唑。毒理学评估发现,当接触浓度为 1.5 毫克/千克-1 的异康唑时,蚯蚓会受到生长抑制作用和组织病理学损伤。进一步研究表明,异康唑的这些毒性作用是通过诱导氧化损伤和影响相关生长的功能基因表达引起的。这些研究成果将进一步加深人们对异丙唑对映体的活性和风险的了解,有助于在农业环境中更安全地使用异丙唑。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Enantioseparation, bioactivity, environmental fate and toxicity of chiral triazole fungicide ipconazole in soil and earthworm
Ipconazole (IPC) is a chiral triazole fungicide and commonly used for disease control in seeds. This study investigated the bioactivity and potential mechanism of ipconazole against pathogenic microorganisms at the chiral perspective. It explored the accumulation behavior of ipconazole enantiomers within the soil-earthworm system and evaluated its toxic effects on earthworms. Bioactivity evaluation revealed that the bioactivity order of ipconazole against three plant pathogens is (−)-1S,2 R,5S-IPC > rac-IPC > (+)-1R,2S,5R-IPC, and the bioactivity of (−)-1S,2 R,5S-IPC is 34.6-129.5 times higher than that of (+)-1R,2S,5R-IPC. Molecular docking found that (−)-1S,2 R,5S-IPC has a stronger binding affinity for the target protein CYP51 to cause activity differences. Accumulation and metabolism studies revealed that (−)-1S,2 R,5S-IPC is more persistent than that of (+)-1R,2S,5R-IPC, and ipconazole was primarily metabolized into hydroxylated ipconazole through hydroxylation in the soil-earthworm system. Toxicological evaluation found growth inhibitory effects and histopathological damage to earthworms at an exposure concentration of 1.5 mg kg-1 ipconazole. Further investigation indicated that these toxic effects of ipconazole were caused by inducing oxidative damage and influencing the functional gene expression of related growth. These research findings will further enhance the understanding of the activity and risks of ipconazole enantiomers, contributing to the safer use of ipconazole in the agricultural environment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


