细胞内微环境在阳离子酞菁高光动力活性中的关键作用
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
为了研究酞菁聚集对其光动力活性的影响,我们合成了一系列含有 4 至 16 个 4-((二乙基甲基铵)甲基)苯氧基取代基的六种阳离子水溶性锌(II)酞菁。根据其结构的不同,酞菁在磷酸盐缓冲溶液中具有从完全组装到单体状态的不同聚集行为。值得注意的是,与在缓冲溶液中的聚集无关,所有研究的酞菁对肿瘤细胞株 MCF-7 和 MDA-MB-231 的光动力效率都非常高,在纳摩尔范围内,IC50(光)从 27 nM 到 358 nM 不等(3.5 J/cm2,660 nm),IC50(暗)/IC50(光)比率高达 ∼3700。正如荧光显微镜所显示的那样,这是由于细胞内聚集的酞菁被分解,形成了单体光活性形式。事实上,在缓冲液中,聚集的酞菁与血清蛋白相互作用,导致非发光聚集体解体,释放出与蛋白质大分子结合的光活性单体。本文章由计算机程序翻译,如有差异,请以英文原文为准。
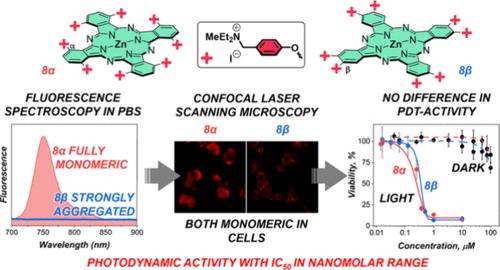
Pivotal Role of the Intracellular Microenvironment in the High Photodynamic Activity of Cationic Phthalocyanines
To investigate the influence of phthalocyanine aggregation on their photodynamic activity, a series of six cationic water-soluble zinc(II) phthalocyanines bearing from four to sixteen 4-((diethylmethylammonium)methyl)phenoxy substituents was synthesized. Depending on their structure, the phthalocyanines have different aggregation behaviors in phosphate buffer solutions ranging from fully assembled to monomeric states. Remarkably, independent of aggregation in buffer, very high photodynamic efficiencies against the tumor cell lines MCF-7 and MDA-MB-231 in the nanomolar range were found for all investigated phthalocyanine, and the IC50(light) varied from 27 to 358 nM (3.5 J/cm2, 660 nm) with IC50(dark)/IC50(light) ratios up to ∼3700. This is due to the intracellular disassembly of aggregated phthalocyanines with the formation of monomeric photoactive forms, as demonstrated by fluorescence microscopy. Indeed, the interaction of aggregated phthalocyanines with serum proteins in a buffer resulted in the disassembly of nonluminescent aggregate species with the release of photoactive monomers bound to protein macromolecules.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


