对苯二胺抗氧化剂的水解:反应机理、预测模型和对水生毒性的潜在影响
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
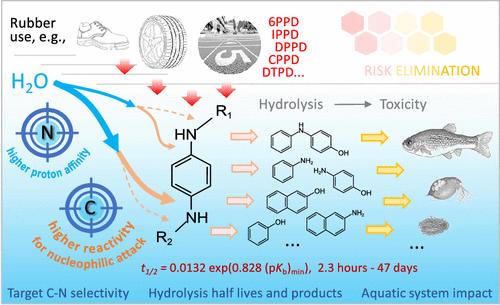
虽然对苯二胺抗氧化剂(PPDs)对水生生态系统构成潜在风险,但由于 PPD C-N 键水解的性质尚未明确,因此其环境持久性和转化仍然模糊不清。在此,我们通过分析 PPD 的水解半衰期、中性 pH 值(pH 值 6.0-7.7)附近的水解产物以及 PPD 中 C-N 键原子的作用,研究了 PPD 的水解模式。水解作用优先针对质子亲和力最强的芳香仲胺 N 和亲核攻击反应性最高的 C-N 的 C 原子。当 N 的最大质子亲和力增加时,水解半衰期(t1/2)会缩短。这些结果得到了理论计算的支持,证明了水解反应由质子从水转移到 N 所推动,并由 C-N 中的 N 被水羟基的芳香亲核取代所补充。利用实验结果和基于原子反应性的预测模型,确定了 60 种 PPD(在环境中监测到的、市售的或正在研究的)在中性 pH 值附近的 t1/2,其变化范围从 2.2 小时到 47 天不等。通过典型的 PPD 证实了模型对初级 C-N 水解的预测。由于阐明了机理并建立了模型,这项研究为聚苯乙烯二异氰酸酯的水解提供了新的见解,并强调了其在划定环境影响方面的重要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Hydrolysis of p-Phenylenediamine Antioxidants: The Reaction Mechanism, Prediction Model, and Potential Impact on Aquatic Toxicity
While p-phenylenediamine antioxidants (PPDs) pose potential risks to aquatic ecosystems, their environmental persistence and transformation remain ambiguous due to the undefined nature of PPD C–N bond hydrolysis. Here, we investigated the hydrolysis patterns of PPDs by analyzing their hydrolysis half-lives, hydrolysis products around neutral pH (pH 6.0–7.7), and the role of atoms within the C–N bonds in PPDs. Hydrolysis preferentially targets the aromatic secondary amine N with the strongest proton affinity and the C atom of C–N with the highest nucleophilic-attack reactivity. The hydrolysis half-life (t1/2) shortens when the maximum proton affinity of N increases. These results are supported by theoretical calculations, demonstrating a hydrolysis reaction propelled by proton transfer from water to N and complemented by aromatic nucleophilic substitution of N in C–N by water hydroxyl. With the experimental results and the atom reactivity-based predictive model, the t1/2 around neutral pH for 60 PPDs (monitored in environment, commercially available, or under investigation) is determined, showing variations ranging from 2.2 h to 47 days. The model prediction of primary C–N hydrolysis is confirmed through typical PPDs. With the elucidated mechanism and developed model, this research provides new insights into PPD hydrolysis, underscoring its significance in delineating environmental impacts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


