揭示与肥胖症代谢健康有关的脂肪群
IF 30.9
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
尽管肥胖症患者的代谢表型存在很大的异质性,但精准医疗仍未被视为肥胖症治疗的标准。脂肪组织(AT)功能障碍是影响代谢性疾病风险变化的最主要因素之一;然而,人们对不同细胞群、细胞类型特异性转录程序和疾病严重程度之间的联系却知之甚少。在这里,我们生成了代谢健康和不健康肥胖者皮下和内脏脂肪组织的综合细胞图谱。通过将单核 RNA 序列数据与大容量转录组学和临床参数相结合,我们发现间皮细胞、脂肪细胞和脂肪细胞祖细胞与代谢性疾病的相关性最强。此外,我们还发现了细胞特异性转录程序,如间皮细胞向间充质表型的转变,这些程序参与了肥胖与代谢性疾病的解耦。这些发现揭示了临床终点的生物学驱动因素,从而提供了宝贵的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
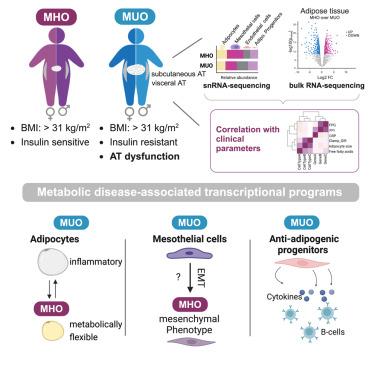
Unveiling adipose populations linked to metabolic health in obesity
Precision medicine is still not considered as a standard of care in obesity treatment, despite a large heterogeneity in the metabolic phenotype of individuals with obesity. One of the strongest factors influencing the variability in metabolic disease risk is adipose tissue (AT) dysfunction; however, there is little understanding of the link between distinct cell populations, cell-type-specific transcriptional programs, and disease severity. Here, we generated a comprehensive cellular map of subcutaneous and visceral AT of individuals with metabolically healthy and unhealthy obesity. By combining single-nucleus RNA-sequencing data with bulk transcriptomics and clinical parameters, we identified that mesothelial cells, adipocytes, and adipocyte-progenitor cells exhibit the strongest correlation with metabolic disease. Furthermore, we uncovered cell-specific transcriptional programs, such as the transitioning of mesothelial cells to a mesenchymal phenotype, that are involved in uncoupling obesity from metabolic disease. Together, these findings provide valuable insights by revealing biological drivers of clinical endpoints.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


