借氢锰催化下酮和仲醇的三戊基化反应
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
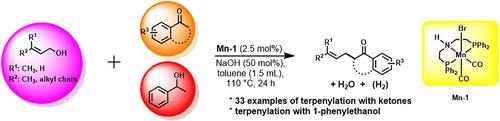
Terpenylation of Ketones and a Secondary Alcohol under Hydrogen-Borrowing Manganese Catalysis
An Earth-abundant Mn–PNP pincer complex-catalyzed terpenylation of cyclic and acyclic ketones and secondary alcohol 1-phenylethanol using isoprenoid derivatives prenol, nerol, phytol, solanesol, and E-farnesol as allyl surrogates is reported. The C–C coupling reactions are green and atom-economic, proceeding via dehydrogenation of alcohols following a hydrogen autotransfer methodology aided by metal–ligand cooperation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


