氢键供体催化的 2,2-二取代环氧化物的一锅转化:功能化富腈衍生物的合成
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们开发了一种具有实际意义的催化多米诺方法,用于在单锅操作中合成高度官能化的吡喃和乙烯-1,1,2-三腈衍生物。在氢键供体(HBD)催化条件下,gem-二氰基烯烃和相应的环氧化物成为反应伙伴。通过无金属有机催化途径,发现该反应在形成连续的 C-C 和 O-C 键以及特殊的 CSp2-CSp 偶联步骤方面具有很高的效率。这一策略已被进一步用于酯类取代的环氧化物,但结果与二氰基环氧化物不同。该工艺在广泛的底物中仍然具有通用性和有效性。事实证明,这种催化方案非常适用于各种底物。这种方法的重要特点是催化剂负载量低、反应条件温和、所有产物的产量都令人满意。此外,活化环氧化物和丙二腈衍生物之间的协同反应模式以及整体原子经济性结果对于该工艺的原创性也非常重要。光谱分析用于验证机理解释。本文章由计算机程序翻译,如有差异,请以英文原文为准。
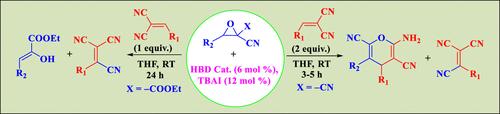
Hydrogen Bond Donor-Catalyzed One-Pot Transformations of 2,2-Disubstituted Epoxides: Synthesis of Functionalized Nitrile-Rich Derivatives
A practically intriguing catalytic domino methodology has been developed for the synthesis of highly functionalized pyran and ethene-1,1,2-tricarbonitrile derivatives in a single-pot operation. The gem-dicyano olefins and the corresponding epoxide were taken as the reactive partners in the presence of a hydrogen bond donor (HBD)-catalyzed condition. The reaction was found to be highly efficient in terms of the formation of sequential C–C and O–C bonds along with an exceptional CSp2–CSp coupling step through a metal-free organocatalytic pathway. This strategy has been further utilized on ester-substituted epoxides, although the results differ from those with gem-dicyano epoxides. The process remains versatile and effective across a wide range of substrates. This catalytic protocol has been proven to be very generalized with varieties of substrate scope. A low catalyst loading, ambient reaction conditions, and satisfactory yields of all of the products are the vital features of this approach. Moreover, the overall atom-economic outcome along with the synergistic reactivity pattern between the activated epoxide and the malononitrile derivatives is also very significant to address the originality of this process. Spectroscopic analysis is utilized to validate the mechanistic interpretation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


