电化学还原铀酰离子的活化和功能化
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
铀氧化态的相互转化促成了涉及该元素的分离和反应方案,并有助于乏核燃料的回收技术。铀物种的氧化还原行为会对这些过程产生影响,但使用电化学方法来驱动铀分子复合物的反应,并从分子角度深入了解电驱动反应的结果,却远未得到应有的重视。在这里,我们展示了铀酰离子 (UO22+) 的电还原可用于促进通常无反应的氧化基团的逐步官能化,外源三苯基硼烷 (BPh3) 可作为温和的亲电子体,避免了对化学还原剂的传统要求。同时进行的电分析、光谱化学和化学反应研究,以及关键还原产物和硼烷化产物的光谱分析结果和 X 射线衍射分析结构数据,证明我们的电化学方法在很大程度上避免了不希望发生的交叉反应和歧化途径;这些通常会影响铀酰官能化化学所需的多组分系统。我们利用联合计算研究绘制了与 U-O 活化相关的变化图,并量化了与关键反应相关的自由能差异。总之,研究结果表明,电化学方法可用于锕系元素分子的选择性相互转化,这让人想起过渡金属氧化还原催化中常用的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
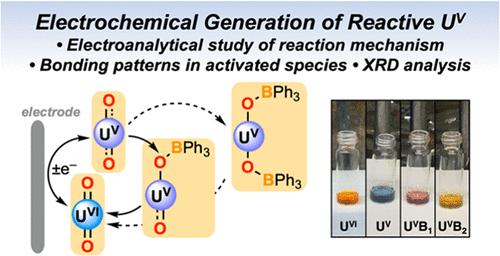
Activation and Functionalization of the Uranyl Ion by Electrochemical Reduction
Interconversion of the oxidation states of uranium enables separations and reactivity schemes involving this element and contributes to technologies for recycling of spent nuclear fuels. The redox behaviors of uranium species impact these processes, but use of electrochemical methods to drive reactions of molecular uranium complexes and to obtain molecular insights into the outcomes of electrode-driven reactions has received far less attention than it deserves. Here, we show that electro-reduction of the uranyl ion (UO22+) can be used to promote stepwise functionalization of the typically unreactive oxo groups with exogenous triphenylborane (BPh3) serving as a moderate electrophile, avoiding the conventional requirement for a chemical reductant. Parallel electroanalytical, spectrochemical, and chemical reactivity studies, supported by spectroscopic findings and structural data from X-ray diffraction analysis on key reduced and borylated products, demonstrate that our electrochemical approach largely avoids undesired cross reactions and disproportionation pathways; these usually impact the multicomponent systems needed for uranyl functionalization chemistry. Joint computational studies have been used to map the changes associated with U–O activation and to quantify the free energy differences related to key reactions. Taken together, the results suggest that electrochemical methods can be used for selective interconversion of molecular actinide species, reminiscent of methods commonly employed in transition metal redox catalysis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


