IF 3.8
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
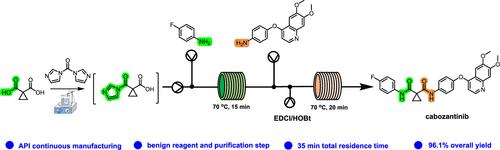
卡博替尼(CBT)是一种小分子药物,已被批准用于治疗甲状腺髓样癌、晚期肾细胞癌和肝细胞癌。以 1,1-环丙烷二羧酸(CBT-1)为起点的两步酰胺化反应是 CBT 的大规模合成路线,但现有工艺存在试剂毒性大、产率低、反应时间长等问题。本研究开发了一种在微反应器中合成 CBT 的两步级联流动工艺。在第一步酰胺化反应中,CDI 被确定为偶联试剂,在优化操作条件后生成了 1-(4-氟苯氨基甲酰基)环丙烷羧酸(CBT-3),产率达 97.5%。然后,筛选出 EDCI/HOBt 作为偶联试剂,在第二步酰胺化反应中获得 CBT,并在微反应器中揭示了可能的反应路径和内在动力学。结果表明,CBT-3 与 EDCI 之间形成离子对是整个反应过程的决定性步骤。最后,两个酰胺化反应在微反应器中级联,在 35 分钟内合成了 CBT,总反应产率达 96.1%。对所开发的微化学系统进行的放大研究表明,CBT 的生产率可达约 5.3 g/h。与现有工艺相比,所使用的试剂/条件更为温和,连续合成的创新方法可为 CBT 的生产提供一种高效的替代方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Two-Step Continuous Synthesis of Cabozantinib in a Microreactor: Mechanism and Kinetics Research of EDCI/HOBt-Mediated Amidation
Cabozantinib (CBT) is a small-molecule pharmaceutical approved for the treatment of medullary thyroid cancer, advanced renal cell carcinoma, and hepatocellular carcinoma. The two-step amidation starting with 1,1-cyclopropanedicarboxylic acid (CBT-1) is a large-scale synthetic route for CBT, but the existing processes suffered from highly toxic reagents, low yields, and long reaction times. In this work, a two-step cascaded flow process was developed to synthesize CBT in a microreactor. In the first amidation step, CDI was determined as the coupling reagent to generate 1-(4-fluorophenylaminoformyl) cyclopropane carboxylic acid (CBT-3) with a yield of 97.5% after optimizing the operating conditions. Then, EDCI/HOBt were screened as the coupling reagents to obtain CBT in the second amidation step, and the possible reaction paths and intrinsic kinetics were revealed in the microreactor. The result indicated that the formation of ion pairs between CBT-3 and EDCI was the rate-determining step in the whole reaction process. In the end, the two amidation reactions were cascaded in a microreactor, and CBT was synthesized with a total reaction yield of 96.1% in 35 min. The scale-up of the developed microchemical system showed that the productivity of CBT can achieve about 5.3 g/h. Compared with the existing processes, milder reagents/conditions were used, and the innovative approach of continuous synthesis can provide an efficient and alternative method for the production of CBT.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


