芒果核生物炭负载多硫化物-绿色合成-纳米零价铁强化水中Cr(VI)的去除:性能与机理
IF 4.3
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
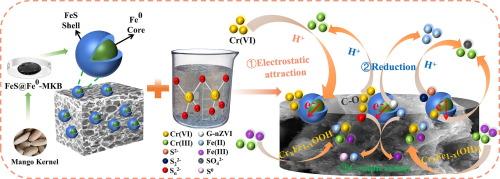
本研究利用废弃的芒果核作为绿色还原剂,残渣作为生物炭前驱体,采用一锅两步法制备了芒果核生物炭支持的多硫化绿色合成纳米级零价铁(FeS@Fe0-MKB),用于去除水中的六价铬。通过引入多硫化钙(CaSx)作为硫化物试剂,Fe0 的抗氧化性和电子转移能力得到了显著提高。S/Fe 摩尔比为 2/1 的复合材料(FeS@Fe0-MKB2)对六价铬的处理效果最好,30 分钟内的去除率达到 99.9%。伪二阶动力学模型和颗粒内扩散模型较好地解释了 FeS@Fe0-MKB2 对六价铬的去除动力学。在 FeS@Fe0-MKB 系统中,硫物种(Sn2-、S2- 和 S22-)、铁物种(Fe0、Fe2+)和生物炭功能基团是关键的电子供体。FeS@Fe0-MKB2 是一种具有令人满意的稳定性和可循环性的复合材料。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Enhanced removal of aqueous Cr(VI) by mango kernel biochar supported polysulfide-green synthesized-nanoscale zero valent iron: Performance and mechanism
This study utilized waste mango kernels as a green reducing agent with the residue serving as the biochar precursor in a one-pot, two-step process to prepare mango kernel biochar supported polysulfide-green synthesized-nanoscale zero valent iron (FeS@Fe0-MKB) for Cr(VI) removal from water. The oxidation resistance and electron transfer capacity of Fe0 were significantly enhanced by the introduction of calcium polysulfide (CaSx) as a sulfide reagent. The composite with S/Fe molar ratio of 2/1 (FeS@Fe0-MKB2) exhibited the greatest efficacy for Cr(VI) treatment, with a removal efficiency of 99.9% in 30 min. The pseudo-second-order kinetic model and intra particle diffusion model provided better explanations for Cr(VI) removal kinetics by FeS@Fe0-MKB2. Sulfur species (Sn2–, S2– and S22–), iron species (Fe0, Fe2+), and biochar functional groups served as pivotal electron donors in the FeS@Fe0-MKB system. FeS@Fe0-MKB2 was a composite with satisfactory stability and cyclability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Science
工程技术-工程:化工
CiteScore
7.50
自引率
8.50%
发文量
1025
审稿时长
50 days
期刊介绍:
Chemical engineering enables the transformation of natural resources and energy into useful products for society. It draws on and applies natural sciences, mathematics and economics, and has developed fundamental engineering science that underpins the discipline.
Chemical Engineering Science (CES) has been publishing papers on the fundamentals of chemical engineering since 1951. CES is the platform where the most significant advances in the discipline have ever since been published. Chemical Engineering Science has accompanied and sustained chemical engineering through its development into the vibrant and broad scientific discipline it is today.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


