非活化烯烃的光子驱动自由基氢膦化反应
IF 11.5
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
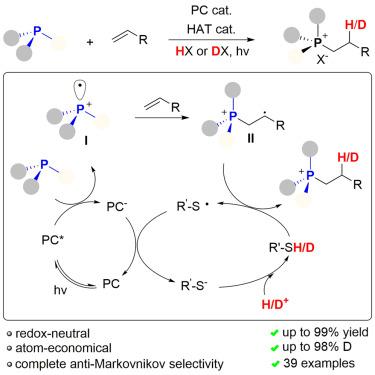
鏻盐广泛应用于有机合成、催化、材料科学和药物化学。烯烃的氢化膦化反应是膦盐合成中最强大、最直接的方法之一。然而,已有的膦-迈克尔反应仅限于电子活化的烯烃,未活化的烯烃没有反应。在此,我们报告了一种光催化和氧化还原中性方案,用于将膦和 CF3COOH 有效地加成到各种未活化的烯烃中,这些烯烃在热化学条件下热力学上是不利的。反应开始时,激发的光催化剂对膦进行单电子氧化,生成膦自由基阳离子(PRC)。PRC 以无动力学障碍的方式加入烯烃。该方法的底物范围很广,既可用于膦,也可用于烯。通过这种与 CF3COOD/D2O 的反应,还可以获得用其他方法难以合成的 β-氚化鏻盐。机理和密度泛函理论(DFT)研究支持 P-C 键形成的自由基加成机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Photon-driven radical hydro-phosphoniumylation of unactivated olefins
Phosphonium salts are widely applied in organic synthesis, catalysis, materials science, and medicinal chemistry. Hydro-phosphoniumylation of alkene is one of the most powerful and straightforward methodologies for phosphonium salt synthesis. However, the established phospha-Michael reaction is limited to electronically activated olefins, and unactivated alkenes are not reactive. Herein, we report a photocatalytic and redox-neutral protocol for the efficient addition of phosphines and CF3COOH to various unactivated olefins, which would be thermodynamically unfavorable under thermochemical conditions. The reaction commences with the generation of a phosphine radical cation (PRC) through the single-electron oxidation of phosphine by an excited photocatalyst. PRC adds to alkene in a kinetically barrierless manner. The method exhibits a broad substrate scope for both phosphines and alkenes. β-Deuterated phosphonium salts, whose synthesis is difficult by other methods, could also be accessed by this reaction with CF3COOD/D2O. Mechanistic and density functional theory (DFT) studies support a radical addition mechanism for P–C bond formation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.50
自引率
6.40%
发文量
0
期刊介绍:
Chem Catalysis is a monthly journal that publishes innovative research on fundamental and applied catalysis, providing a platform for researchers across chemistry, chemical engineering, and related fields. It serves as a premier resource for scientists and engineers in academia and industry, covering heterogeneous, homogeneous, and biocatalysis. Emphasizing transformative methods and technologies, the journal aims to advance understanding, introduce novel catalysts, and connect fundamental insights to real-world applications for societal benefit.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


