通过分子间二硫键的共价和正交凝聚素-Dockerin相互作用实现超稳定纤维素酶组装
IF 3.8
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
纤维素生物燃料是化石燃料的可持续替代品,但纤维素酶的高成本阻碍了其发展。恒温纤维素体可在高温条件下发挥作用,通过使用凝聚素-对接素相互作用来共聚超恒温纤维素酶,从而提高反应速率并降低冷却成本。由于凝聚素-对接素相互作用的非共价性质,纤维素体的稳定性一直被限制在 75 °C。我们的研究利用计算设计和快速筛选,在同一种凝聚素和对接素之间引入了两种不同的分子间二硫桥,形成了两种二硫凝聚素-对接素对。这两对二硫键都能经受 100 °C 和变性条件的考验。此外,这两个二硫桥还保持了正交性,从而增加了正交凝聚素-对接素相互作用的数量。最后,在纤维素酶的最佳温度 80 °C,二硫组装使二价纤维素体的活性比非共价的纤维素体提高了 26%。这些二硫键合蛋白-Dockerin相互作用可用作构建可承受极端温度的共价蛋白质复合物的构件。本文章由计算机程序翻译,如有差异,请以英文原文为准。
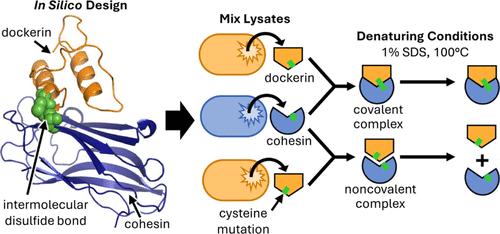
Covalent and Orthogonal Cohesin–Dockerin Interactions Enabled by Intermolecular Disulfide Bonds for Hyperthermostable Cellulase Assembly
Cellulosic biofuel represents a sustainable alternative to fossil fuels, yet high cellulase costs hinder its development. Thermostable cellulosomes, which function at elevated temperatures, increase reaction rates and reduce cooling costs by using cohesin–dockerin interactions to colocalize hyperthermostable cellulases. Due to the noncovalent nature of the cohesin–dockerin interaction, cellulosome stability has been limited to 75 °C. Our study leverages computational design and rapid screening to introduce two different intermolecular disulfide bridges between the same cohesin and dockerin, creating two disulfide cohesin–dockerin pairs. Both disulfide pairs withstood 100 °C and denaturing conditions. Furthermore, the two disulfide bridges retained their orthogonality, expanding the number of orthogonal cohesin–dockerin interactions. Finally, at the cellulase optimal temperature of 80 °C, disulfide assembly improved the activity of a bivalent cellulosome by 26% compared to that of its noncovalent counterpart. These disulfide cohesin–dockerin interactions can be used as building blocks to construct covalent protein complexes that can endure extreme temperatures.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


