IF 7.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
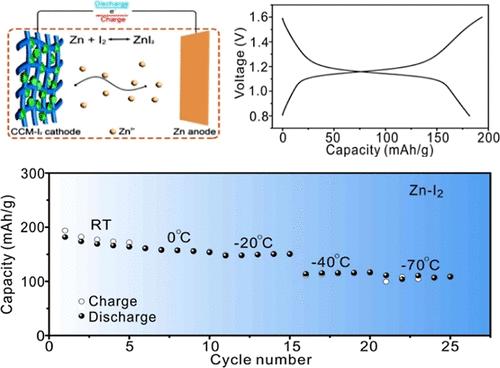
锌离子水电池(AZIBs)因其环保、低价和高安全性而备受关注。然而,水溶液电解质的液固转换温度较高,这与通过氢键(HB)连接的水网络密切相关,严重制约了 AZIB 的应用。另一个关键技术问题是探索与低温水性电解质兼容的适当电极材料。为了确保电池在低温条件下正常工作,开发了一种含有多组分高氯酸盐(Zn、Ca、Mg、Li)ClO4 的高熵电解质(HEE)。量热分析表明,这种高熵电解质具有极低的液晶转变温度(-114 °C)。利用拉曼光谱、傅里叶变换红外光谱和核磁共振光谱进行的结构表征表明,在水性电解质中引入多组分高氯酸盐,通过形成 M----(H2O)n----ClO4--(M 为 Zn2+、Ca2+、Mg2+ 或 Li+)构型打破了最初的水网络,因此 HEE 即使在 -70 ℃ 下也不会冻结。原位粘度测量表明,HEE 在 -70 °C 时的粘度为 13.8 mPa S。电化学测量结果表明,HEE 在 25 °C 时的离子电导率为 22.6 mS cm-1,在 -70 °C 时的离子电导率为 2.7 mS cm-1。系统地研究了 HEE 与不同电极材料的兼容性,特别是本研究中预插入阳离子的氧化钒(KVO)和含碘的碳复合材料(CCM/I2),电化学测量结果表明 HEE 显示了电池系统的选择性。KVO|HEE|Zn 电池在室温下的循环稳定性较差(在 5.0 A g-1 条件下循环 5,000 次后只有 33 mA h g-1),而 CCM-I2|HEE|Zn 电池在第一次循环 100 mA g-1 时的容量为 182 mA h g-1,循环性能优越(在 5.0 A g-1 条件下循环 5,000 次后 102 mA h g-1)。低温电化学测量结果表明,与二元电解质体系(Zn, 3Ca)ClO4)相比,含有 HEE 的电池体系在 -70 °C 下具有更强的电化学性能。这项研究揭示了电极/电解质适应性对 AZIB 电化学性能的重要影响,并为采用多组分高熵策略构建低温电解质提供了宝贵的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Selective Compatibility of High-Entropy Electrolytes for Low-Temperature Aqueous Zinc–Iodine Batteries
Aqueous zinc-ion batteries (AZIBs) have attracted massive interest on account of their environmental friendliness, low price, and high security. Nevertheless, the application of AZIBs is seriously constrained by the high liquid–solid transition temperature of aqueous electrolytes, which is strongly related to the water network connected through hydrogen bonds (HBs). Another critical technical issue is to explore the appropriate electrode material compatible with a low-temperature aqueous electrolyte. In order to ensure the battery works properly at low-temperature conditions, a high-entropy electrolyte (HEE) with multicomponent perchlorate salts, (Zn, Ca, Mg, Li)ClO4, is developed. The calorimetric analysis indicates that the HEE exhibits an extremely low liquid–glass transition temperature (−114 °C). Structural characterizations using Raman, FTIR, and NMR spectroscopy indicate that the introduction of multicomponent perchlorate salts into the aqueous electrolyte breaks the initial water network by the formation of M···(H2O)n···ClO4– (M is Zn2+, Ca2+, Mg2+ or Li+) configurations, and the HEE therefore remains unfrozen even at −70 °C. The in situ viscosity measurement indicates the HEE has a viscosity of 13.8 mPa S at −70 °C. The electrochemical measurements indicate that the ionic conductivity of the HEE is 22.6 mS cm–1 at 25 °C and 2.7 mS cm–1 at −70 °C, and it has excellent electrochemical compatibility with Zn metal upon cycling Zn||Zn symmetric cells. The compatibility of the HEE and different electrode materials, particularly vanadate oxide with preinserted cations (KVO) and a carbon composite material with iodine (CCM/I2) in this study, is systematically investigated, and the results of electrochemical measurements indicate the HEE shows the selectivity of battery systems. The KVO|HEE|Zn battery exhibits poor cycling stability at room temperature (only 33 mA h g–1 after 5,000 cycles at 5.0 A g–1), while the CCM-I2|HEE|Zn battery displays a capacity of 182 mA h g–1 at 100 mA g–1 in the first cycle and superior cycling performances (102 mA h g–1 after 5,000 cycles at 5.0 A g–1). Low-temperature electrochemical measurements demonstrate that the battery system with the HEE exhibits enhanced electrochemical performances at −70 °C when compared with the binary electrolyte system (Zn, 3Ca)ClO4. This work reveals the significance of electrode/electrolyte adaptability on the electrochemical performances of AZIBs and provides valuable insights for constructing low-temperature electrolytes using a multicomponent high-entropy strategy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


