甲醇和水低温制氢过程中的铂电子调控
IF 7.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
甲醇的水相重整(APRM)反应为氢(H2)的储存和运输提供了一种潜在的方法。然而,由于铂纳米催化剂活化 H2O 的能力有限,因此需要较高的反应温度(200 °C)才能通过 APRM 反应高效生成 H2。在这里,铂纳米催化剂的电子密度受 Al2O3 支持物相的调节。机理分析表明,晶格边缘间距较大的 α-Al2O3 载体可提高铂纳米催化剂的电子密度,从而实现 H2O 的有效吸附和活化。因此,Pt/α-Al2O3 催化剂在 30 °C 下通过 APRM 反应生成 H2 的 TOF 值为 69.8 h-1。值得注意的是,这一 H2 生成率甚至低于之前最先进的均相催化剂。这一发现为灵活利用氢气提供了一条前景广阔的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
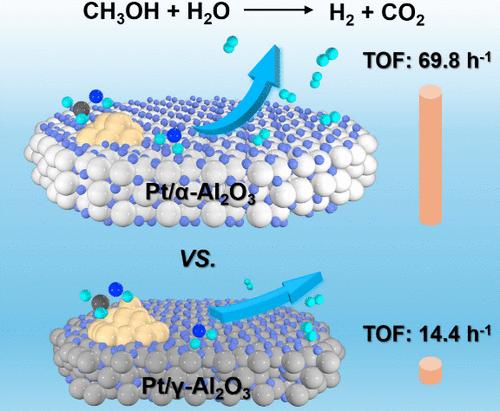
Electronic Regulation of Pt for Low-Temperature Hydrogen Generation from Methanol and Water
The aqueous-phase reforming of the methanol (APRM) reaction provides a potential approach for hydrogen (H2) storage and transportation. However, the limited capacity of Pt nanocatalysts for H2O activation leads to the drawback of requiring high reaction temperatures (>200 °C) to achieve efficient H2 generation through the APRM reaction. Herein, the electronic density of Pt nanocatalysts has been regulated by the phase of the Al2O3 supports. Mechanism analysis revealed that the α-Al2O3 supports with larger lattice fringe spacing resulted in an enhanced electronic density of Pt nanocatalysts, thereby enabling the effective adsorption and activation of H2O. Consequently, the Pt/α-Al2O3 catalysts exhibited a TOF value of 69.8 h–1 at 30 °C for H2 generation via APRM reaction. Notably, this H2 generation rate even suppressed that achieved by previous state-of-the-art homogeneous catalysts. This finding presents a promising avenue toward flexible hydrogen utilization.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


