IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
芳基硼酸在可见光下的有氧氧化被广泛用于合成酚类物质,但由于需要使用牺牲性电子供体,其良性和可持续性大打折扣。在这里,我们首次展示了芳基硼酸的非牺牲性光催化羟基化反应。通过将贫电子底物转化为富电子底物,光催化有氧氧化的进行机制完全不同于以往的机制,包括通过可见光诱导的单电子转移到光催化剂上,将芳基-B 底物直接氧化为芳基自由基。该方案不仅无需牺牲电子供体,还能在水中进行高效反应。它适用于各种光催化剂,无论是均相还是异相。这项工作为合成苯酚的传统方法提供了一种绿色替代方法,从中获得的启示可能为涉及芳基自由基的有机光合作用开辟新的前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
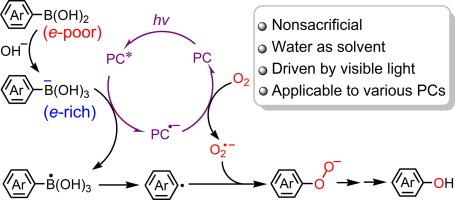
Turning substrates from electron-poor to electron-rich for nonsacrificial aerobic hydroxylation under visible light
Aerobic oxidation of arylboronic acids under visible light has been intensively explored for synthesis of phenols, for which the need for sacrificial electron-donor agents detracts from the benignancy and sustainability. Here we present the first demonstration of nonsacrificial photocatalytic hydroxylation of arylboronic acids. By turning the electron-poor substrates to electron-rich, the photocatalytic aerobic oxidation proceeds through a mechanism completely different from previous ones, involving the direct oxidation of the aryl-B substrates to aryl radicals through visible-light-induced single-electron transfer to photocatalysts. The protocol not only obviates the need for sacrificial electron donors but also allows efficient reactions in water. It is applicable to various photocatalysts, either homogeneous or heterogeneous. The work provides a green alternative to the traditional methods for synthesis of phenols, and the insight gained from it may open new perspectives for organic photosynthesis that involve aryl radicals.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


