用于 4.6 V 金属锂电池的弱配位磺酰胺电解质的溶解结构工程设计
IF 19.3
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
我们合成了一系列三氟甲磺酰胺溶剂,并系统地控制了其 N 端环的大小(4-6),以调整其立体和电子特性,从而增强接触离子对,形成阴离子衍生的固体电解质界面(SEI),并与 NMC811 阴极兼容。电解质的比较分析表明,1.6 M LiFSI 1-氮杂环丁烷三氟甲磺酰胺(AzTFSA)电解质是立体效应和电子效应的理想结合,具有高达 5 V 的高氧化稳定性,在 1 mA cm-2 和 1 mAh cm-2 的铜锂半电池中库仑效率达到 99.2%。相应的全电池使用 20 μm 的锂箔与 NCM811 阴极配对,正负容量比 (N/P) 为 2.5,在 0.5C 下循环 150 次后,容量保持率达到 80%。即使在 4.6 V 的高充电截止电压下,Li|NCM811 全电池在 0.5C 下循环 100 次后仍能保持 92% 的容量。本文章由计算机程序翻译,如有差异,请以英文原文为准。
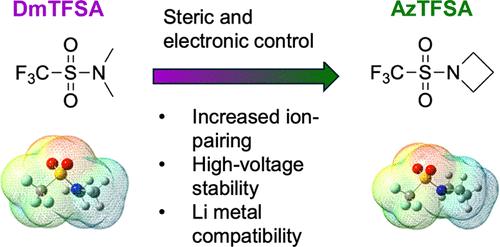
Solvation Structure Engineering of Weakly Coordinating Sulfonamide Electrolytes for 4.6 V Lithium Metal Batteries
A series of trifluoromethanesulfonamide solvents were synthesized with systematically controlled ring size (4–6) at the N-terminal to tune their steric and electronic properties to realize enhanced contact ion pairs for the formation of an anion-derived solid-electrolyte interface (SEI) and compatibility with the NMC811 cathode. Comparative analyses of electrolytes revealed that the 1.6 M LiFSI 1-azetidine trifluoromethanesulfonamide (AzTFSA) electrolyte presents the ideal combination of steric and electronic effects along with high oxidation stability up to 5 V and a Coulombic efficiency of 99.2% in Cu–Li half-cells at 1 mA cm–2 and 1 mAh cm–2. The corresponding full cells using 20 μm of Li foil paired with the NCM811 cathode by a negative and positive capacity ratio (N/P) of 2.5, achieve 80% capacity retention after 150 cycles at 0.5C. Even at a high charge cutoff voltage of 4.6 V, the Li|NCM811 full cell still realizes 92% retention at 0.5C after 100 cycles.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Energy Letters
Energy-Renewable Energy, Sustainability and the Environment
CiteScore
31.20
自引率
5.00%
发文量
469
审稿时长
1 months
期刊介绍:
ACS Energy Letters is a monthly journal that publishes papers reporting new scientific advances in energy research. The journal focuses on topics that are of interest to scientists working in the fundamental and applied sciences. Rapid publication is a central criterion for acceptance, and the journal is known for its quick publication times, with an average of 4-6 weeks from submission to web publication in As Soon As Publishable format.
ACS Energy Letters is ranked as the number one journal in the Web of Science Electrochemistry category. It also ranks within the top 10 journals for Physical Chemistry, Energy & Fuels, and Nanoscience & Nanotechnology.
The journal offers several types of articles, including Letters, Energy Express, Perspectives, Reviews, Editorials, Viewpoints and Energy Focus. Additionally, authors have the option to submit videos that summarize or support the information presented in a Perspective or Review article, which can be highlighted on the journal's website. ACS Energy Letters is abstracted and indexed in Chemical Abstracts Service/SciFinder, EBSCO-summon, PubMed, Web of Science, Scopus and Portico.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


