用于时空控制 CRISPR-Cas9 基因编辑和协同光动力疗法的近红外光遗传纳米系统
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
在时空分辨率水平上控制 CRISPR/Cas9 基因编辑,尤其是在体内应用方面,仍然是一个巨大的挑战。在这里,我们开发了一种近红外(NIR)光激活纳米光子系统(UCPP),用于控制 CRISPR-Cas9 基因编辑和协同光动力疗法(PDT)。掺杂镧系元素的上转换纳米粒子不仅被用作细胞内输送质粒的载体,还可作为纳米换能器,将近红外光(980 纳米)转换为可见光,并在 460 纳米和 650 纳米处发射,从而分别同时激活基因编辑和光动力疗法过程。这种独特的设计不仅实现了对缺氧诱导因子 1α 的光控精确基因编辑,将脱靶效应降至最低,有效改善了肿瘤部位的缺氧状态,而且还促进了深层光导治疗过程,产生了协同抗肿瘤效应。这种光遗传学可激活的 CRISPR-Cas9 纳米系统在空间控制的体内基因编辑和癌症靶向治疗方面具有巨大潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
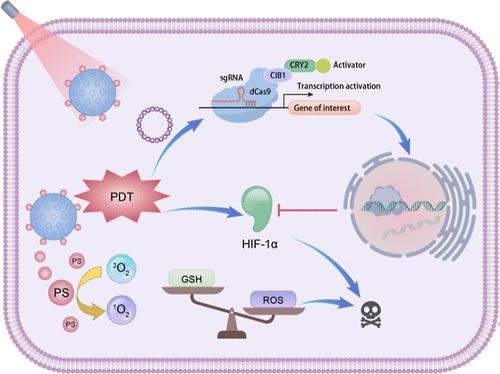
Near-Infrared Optogenetic Nanosystem for Spatiotemporal Control of CRISPR-Cas9 Gene Editing and Synergistic Photodynamic Therapy
Controlling CRISPR/Cas9 gene editing at the spatiotemporal resolution level, especially for in vivo applications, remains a great challenge. Here, we developed a near-infrared (NIR) light-activated nanophotonic system (UCPP) for controlled CRISPR-Cas9 gene editing and synergistic photodynamic therapy (PDT). Lanthanide-doped upconversion nanoparticles are not only employed as carriers for intracellular plasmid delivery but also serve as the nanotransducers to convert NIR light (980 nm) into visible light with emission at 460 and 650 nm, which could result in simultaneous activation of gene editing and PDT processes, respectively. Such unique design not only achieves light-controlled precise gene editing of hypoxia-inducible factor 1α with minimal off-target effect, which effectively ameliorates the hypoxic state at tumor sites, but also facilitates the deep-seated PDT process with synergistic antitumor effect. This optogenetically activatable CRISPR-Cas9 nanosystem holds great potential for spatially controlled in vivo gene editing and targeted cancer therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


