聚 l-乳酸纳米纤维膜能有效抑制肝癌细胞生长并防止术后残余癌复发
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
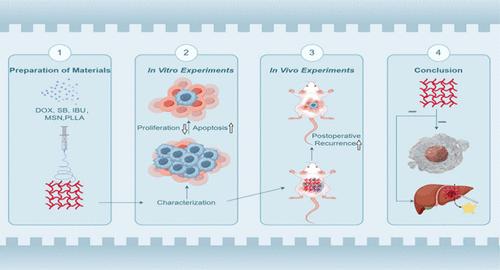
电纺纳米载体系统被广泛应用于医疗领域,具有封装多种药物和减轻并发症的能力。盐酸多柔比星(DOX)是肝癌患者常用的化疗药物。碳酸氢钠(SB)可中和酸性肿瘤微环境,而布洛芬(IBU)则可减轻炎症因子的产生。这三种常用药物的联合使用有助于提高抗肿瘤疗效和预防复发。将抗肿瘤药物 DOX 加入 MSN 中,然后在聚乳酸(PLLA)电纺丝溶液中与 IBU 共同分散,再使用 SB 进行酸敏化,制备出复合纤维膜。利用透射电子显微镜和扫描电子显微镜对所制备的膜进行了表征。评估了这种纤维膜的毒性作用及其对肿瘤细胞的促凋亡作用,以及细胞增殖相关因子、免疫/炎症因子和凋亡相关因子的表达。免疫组化和 HE 染色证实了其抑制术后残留癌复发的能力,且不会对重要器官造成毒性。PLLA-MSN@DOX-SB-IBU纳米纤维膜不仅能减轻与DOX相关的心脏毒性,还能抑制肿瘤细胞增殖并改善肿瘤微环境,具有显著的抗肿瘤功效。此外,它还能有效防止术后残留癌症的复发,同时具有良好的生物相容性。PLLA-MSN@DOX-SB-IBU纳米纤维膜在阻碍肝细胞癌的进展和减轻肝细胞癌手术治疗后残留癌的复发方面具有巨大的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Poly l-Lactic Acid Nanofiber Membrane Effectively Inhibits Liver Cancer Cells Growth and Prevents Postoperative Residual Cancer Recurrence
Electrospun nanocarrier systems, widely employed in the medical field, exhibit the capability to encapsulate multiple drugs and mitigate complications. Doxorubicin hydrochloride (DOX) represents a frequently utilized chemotherapeutic agent for liver cancer patients. Sodium bicarbonate (SB) serves to neutralize the acidic tumor microenvironment, while ibuprofen (IBU) attenuates inflammatory factor production. The combination of these three commonly used drugs facilitates antitumor efficacy and relapse prevention. Composite fibrous membranes were prepared by incorporating the antitumor drug DOX into MSN, which was then codispersed with IBU in a poly l-lactic acid (PLLA) electrospinning solution after acid sensitization using SB. The resulting membrane was characterized using transmission electron microscopy and scanning electron microscopy. The toxic effect of this fibrous membrane and its pro-apoptotic effect on tumor cells were evaluated, along with the expression of cell proliferation-related factors, immune/inflammatory factors, and apoptosis-related factors. Immunohistochemistry and HE staining confirmed its ability to inhibit recurrence of postoperative residual cancer without causing toxicity to vital organs. The PLLA-MSN@DOX-SB-IBU nanofibrous membrane not only mitigates the cardiotoxicity associated with DOX but also inhibits tumor cell proliferation and enhances the tumor microenvironment, demonstrating significant antitumor efficacy. Furthermore, it effectively prevents the recurrence of residual cancer postsurgery while exhibiting excellent biocompatibility. The PLLA-MSN@DOX-SB-IBU nanofibrous membrane demonstrates significant potential in impeding the progression of hepatocellular carcinoma and mitigating the recurrence of residual cancer following surgical intervention for hepatocellular carcinoma.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


