Fe杂质反应对MgFeB2O5电化学性能的影响
IF 7.2
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
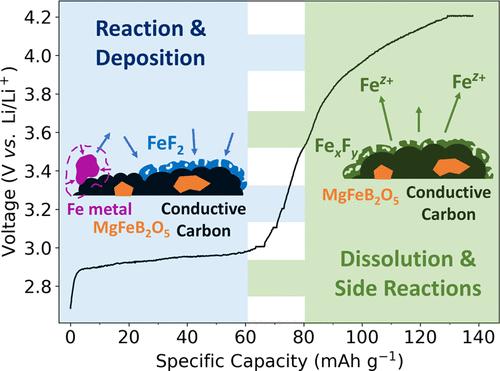
我们研究了作为可充电镁离子电池潜在阴极材料的镁铁硼酸盐 MgFeB2O5。同步辐射粉末 X 射线衍射和 Mössbauer 光谱证实了它的成功合成和高自旋铁(II)态铁的稳定。针对锂金属阳极的初步电化学测试得出的首次充电容量接近理论值(147.45 mAh-g-1),表明 MgFeB2O5 是一种很有前途的阴极候选材料。然而,包括扫描电子显微镜能量色散 X 射线(SEM-EDS)分析、操作性 X 射线吸收近缘光谱(XANES)和莫斯鲍尔光谱在内的多模态分析表明,其中不存在任何铁氧化还原反应。相反,我们认为所观察到的容量来源于少量(4-7 wt%)铁金属杂质的不可逆反应。这些发现突出表明,在评估新型镁阴极材料的性能时,需要采用不同的表征技术,因为初期循环的良好表现可能是由竞争性副反应而不是镁(脱)插层引起的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Role of Fe Impurity Reactions in the Electrochemical Properties of MgFeB2O5
We investigate magnesium–iron pyroborate MgFeB2O5 as a potential cathode material for rechargeable magnesium-ion batteries. Synchrotron powder X-ray diffraction and Mössbauer spectroscopy confirm its successful synthesis and iron stabilization in the high-spin Fe(II) state. Initial electrochemical testing against a lithium metal anode yields a first charge capacity near the theoretical value (147.45 mAh·g–1), suggesting MgFeB2O5 as a promising cathode candidate. However, multimodal analyses, including scanning electron microscopy energy-dispersive X-ray (SEM-EDS) analysis, operando X-ray absorption near edge spectroscopy (XANES), and Mössbauer spectroscopy, reveal the absence of any Fe redox reactions. Instead, we propose that the source of the observed capacity involves the irreversible reaction of a small (4–7 wt%) Fe metal impurity. These findings highlight the need for diverse characterization techniques in evaluating the performance of new Mg cathode materials, since promising initial cycling may be caused by competing side reactions rather than Mg (de)intercalation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


