IF 7.2
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
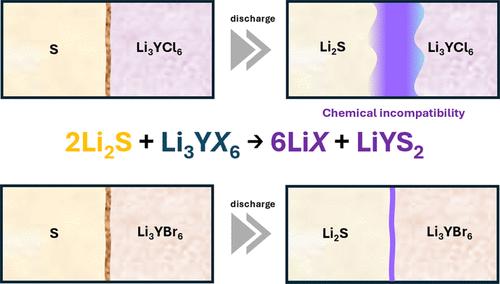
在固态电池中利用富含地球的高容量硫是一种很有前途的策略,可以避免使用稀有过渡金属,提高可实现的比能量。然而,仍然存在许多挑战。阴极复合材料内部的传输限制,特别是充电期间硫化物电解质的传输限制,已被确定为固态锂-S 电池的主要降解机制。这种降解与电解质氧化以及阴极复合材料有效离子导电性的降低有关。受卤化物电解质足够高的氧化稳定性的启发,我们在这项工作中研究了它们与固态锂-S 电池的兼容性。除了卤化物基复合电极的可循环性之外,我们还探讨了卤化物与导电添加剂接触时的电化学稳定性、复合电极中快速离子传输的稳定窗口、与硫活性材料(如 S 和 Li2S)的化学兼容性。我们采用了三种卤化物作为模型电解质:Li3InCl6、Li3YCl6 和 Li3YBr6。尽管 Li3InCl6 具有很高的氧化稳定性,但由于电解质的还原作用,它表现出快速降解。与 Li3YCl6 的复合材料由于化学不相容,特别是与 Li2S 的不相容,导致在界面上形成 LiYS2,从而失去了容量。相比之下,Li3YBr6 表现出更优越的性能,在 20 个循环中保持了 1100 mAh gS-1 的容量(根据阴极材料中的硫含量进行归一化)。这项研究阐明了基于卤化物的固态锂-S 电池的降解机制,并提出了缓解化学不相容问题的潜在设计策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Compatibility of Halide Electrolytes in Solid-State Li–S Battery Cathodes
The utilization of earth-abundant and high-capacity sulfur in solid-state batteries presents a promising strategy to circumvent the use of rare transition metals and enhance achievable specific energy. However, numerous challenges remain. The transport limitation within the cathode composite, particularly with sulfide electrolytes during charging, has been identified as a major degradation mechanism in solid-state Li–S batteries. This degradation is linked to electrolyte oxidation and a concomitant reduction in the effective ionic conductivity of the cathode composite. Inspired by the sufficiently high oxidation stability of halide-based electrolytes, we investigated their compatibility with solid-state Li–S batteries in this work. The electrochemical stability of halides in contact with conductive additives, the stability window of fast ion transport in the composite electrodes, and chemical compatibility with sulfur-active materials (e.g., S and Li2S), in addition to the cyclability of the halide-based composite electrodes, are explored. Three halides were employed as model electrolytes: Li3InCl6, Li3YCl6, and Li3YBr6. Despite its high oxidation stability, Li3InCl6 exhibited rapid degradation due to electrolyte reduction. The composite with Li3YCl6 lost its capacity because of chemical incompatibility, especially with Li2S, resulting in the formation of LiYS2 at the interface. In contrast, Li3YBr6 demonstrated superior performance, maintaining a capacity of 1100 mAh gS–1 for 20 cycles (normalized to the sulfur content in the cathode material). This study elucidates the degradation mechanisms of halide-based solid-state Li–S batteries and proposes potential design strategies to mitigate chemical incompatibility issues.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


