变废为宝:基于反应物循环的酶激活 DNA 传感器可对目标细胞的微 RNA 进行空间选择性成像
IF 6
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
背景微RNA(miRNA)在癌细胞中的放大成像对于了解潜在的病理过程至关重要。合成催化 DNA 电路是 miRNA 成像的关键工具。然而,现有的催化 DNA 电路大多无法在细胞和体内实现反应物的循环操作。此外,肿瘤部位 miRNA 的特异性成像也是一个挑战。受 "变废为宝 "的启发,我们在此报告了一种基于嘌呤/嘧啶内切酶 1(APE1)激活的反应物循环催化 DNA 电路的用于靶细胞中 miRNA 成像的 DNA 传感器。在 APE1 的存在下,AP 位点发生水解,从而激活传感活性,并在 miRNA 的辅助下触发链置换反应(SDR)。在这种催化 DNA 电路中,当双链废品还原成活性成分时,下一个反应周期就可以启动,这使得它只需消耗燃料 DNA 而无需耗尽反应物,就能连续进行。值得注意的是,脂质体在克服核酸递送的生物障碍方面发挥着重要作用。与传统的催化 DNA 电路相比,该 DNA 传感器可在体内实施放大 miRNA 成像策略,在 APE1 的协助下减少肿瘤外信号,并增强肿瘤与正常组织的对比度。其次,实现了细胞和动物体内 miRNA 图像的高空间选择性。第三,提高了体外和体内成像的信噪比。最后,我们的策略为开发更高效、更有选择性的 DNA 电路提供了一种自动但通用的方法,这种 DNA 电路能够区分癌细胞和正常细胞中的 miRNA,有望在癌症诊断中发挥重要作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
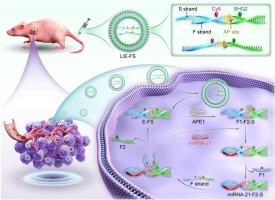

Turning waste into wealth: Enzyme-activated DNA sensor based on reactant recycle for spatially selective imaging microRNA toward target cells
Background
Amplified imaging of microRNA (miRNA) in cancer cells is essential for understanding of the underlying pathological process. Synthetic catalytic DNA circuits represent pivotal tools for miRNA imaging. However, most existing catalytic DNA circuits can not achieve the reactant recycling operation in cells and in vivo. Additionally, specificity miRNA imaging in tumor site also is a challenge. Herein, inspired by “turning waste into wealth”, we report a DNA sensor for imaging of miRNA in target cells based on apurinic/apyrimidinic endonuclease 1 (APE1)-activated reactant recycling catalytic DNA circuit.
Results
The sensing function of the DNA sensor is suppressed firstly through engineering an apurinic/apyrimidinic (AP) site. In the presence of APE1, the AP site undergoes hydrolysis, thereby activating sensing activity and triggering the strand displacement reaction (SDR) under miRNA assistance. In this catalytic DNA circuit, the next reaction cycle can be initiated when the duplex strand waste products are reverted into active components, which allows it to be performed continuously just consuming fuel DNA without depleting the reactant. Noteworthy, the liposome plays important role in overcome biological barriers for nucleic acid delivery. The amplified miRNA imaging strategy is carried out in vivo by this DNA sensor with reducing off-tumor signal under assistance with APE1, and enhances tumor-to-normal tissue contrast compared with traditional catalytic DNA circuit.
Significance and novelty
Firstly, APE1-activated reactant recycling catalytic DNA circuit is developed. Secondly, miRNA image in cell and in animal is achieved with high spatial selectivity. Thirdly, the signal-to-background ratio for imaging is improved in vitro and in vivo. Lastly, our strategy provides an automatic yet versatile approach for the development of more efficient and selective DNA circuits capable of differentiating miRNA in cancer cells from those in normal cells, promising to be valuable in cancer diagnosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytica Chimica Acta
化学-分析化学
CiteScore
10.40
自引率
6.50%
发文量
1081
审稿时长
38 days
期刊介绍:
Analytica Chimica Acta has an open access mirror journal Analytica Chimica Acta: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Analytica Chimica Acta provides a forum for the rapid publication of original research, and critical, comprehensive reviews dealing with all aspects of fundamental and applied modern analytical chemistry. The journal welcomes the submission of research papers which report studies concerning the development of new and significant analytical methodologies. In determining the suitability of submitted articles for publication, particular scrutiny will be placed on the degree of novelty and impact of the research and the extent to which it adds to the existing body of knowledge in analytical chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


